A Primer on Stoichiometry

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
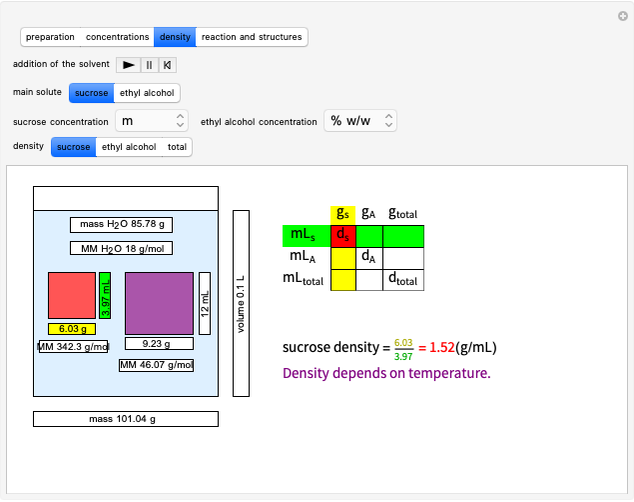
Stoichiometry can be inspired by the philosophical dictum of Parmenides: ex nihilo nihil facit (nothing comes from nothing). Stoichiometry is based on the law of conservation of mass in a chemical reaction (Lavoisier's law). We also show how to convert from moles to several other chemical units.
[more]
Contributed by: A. Ratti, D. Meliga, L. Lavagnino and S. Z. Lavagnino (June 2021)
Open content licensed under CC BY-NC-SA
Snapshots
Details
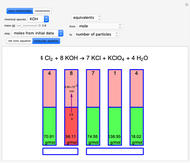
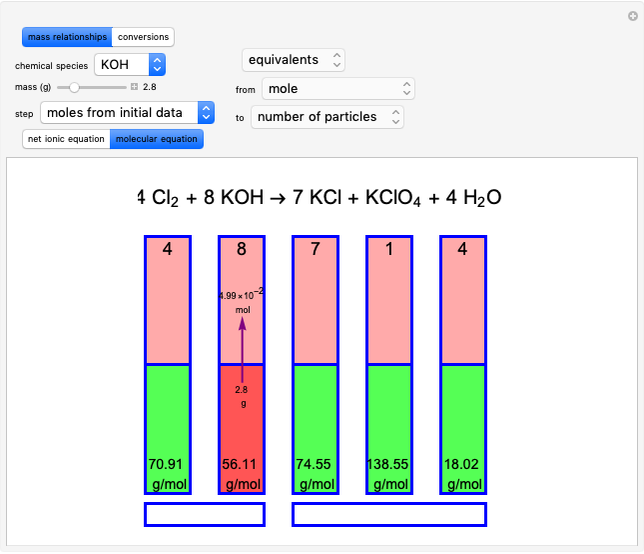
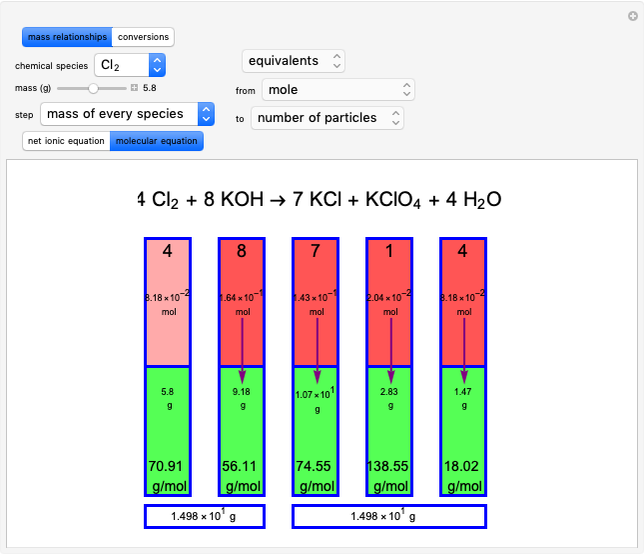
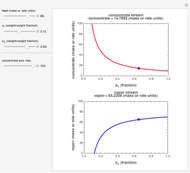
Snapshot 1: first step, moles from the initial mass of 
Snapshot 2: final step, every mass is calculated from the initial mass of 
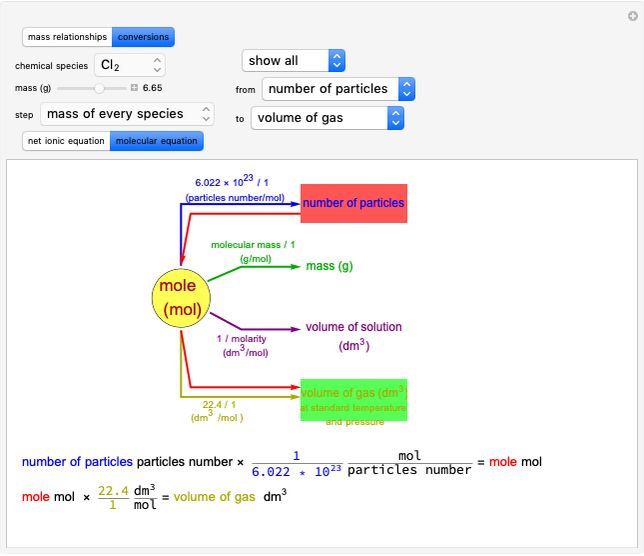
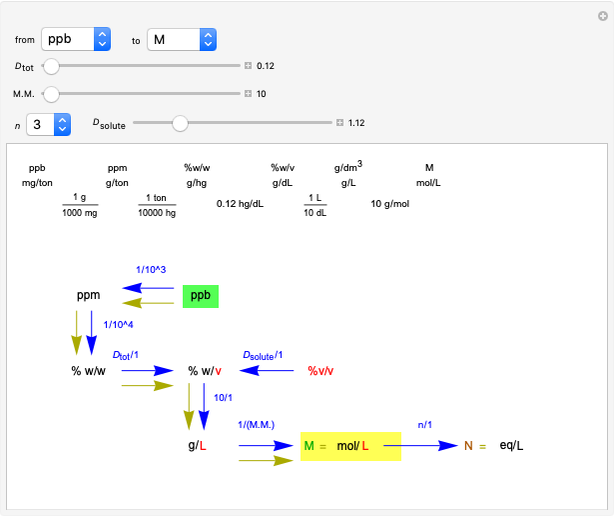
Snapshot 3: conversion factors from mass (grams) to volume of solution ( )
)
Reference
[1] P. M. Lausarot and G. A. Vaglio, Stechiometria per la Chimica generale, Padova, Italy: Piccin-Nuova Libraria S.p.A., 2005.
Permanent Citation