Absorption of Acetone in Air Using Water

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
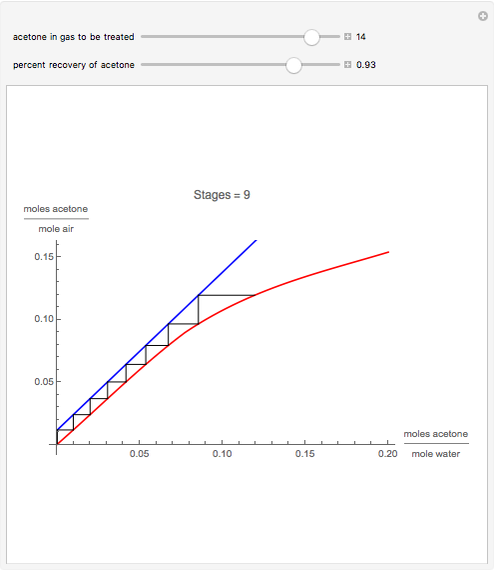
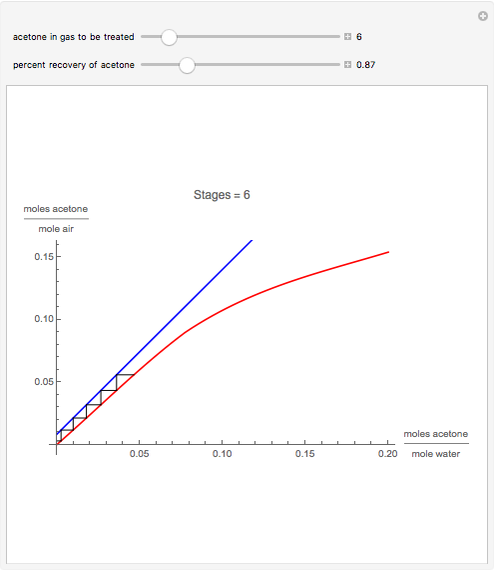
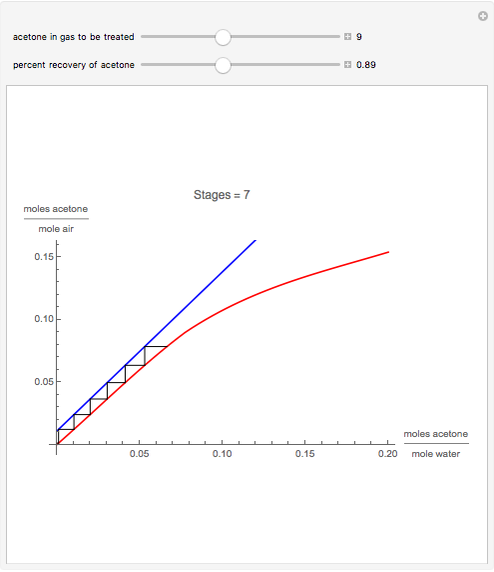
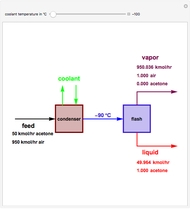
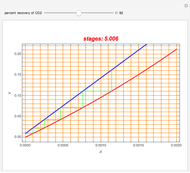
Gas absorption is a very ubiquitous unit operation in any chemical plant. It has in common with liquid-liquid extraction that there are two carrier streams and one solute to be partitioned between them. Here, the McCabe and Thiele graphical method is used to study the separation of acetone from air, using water to absorb acetone from air. You can use the sliders to select the initial amount of acetone in air and the percent recovery of acetone (i.e. how much acetone will be extracted from air using water). The minimum  ratio is determined and the operating line's slope is selected to be a ratio
ratio is determined and the operating line's slope is selected to be a ratio  . Stages are alternately stepped off using the equilibrium curve (red curve) and the operating line (blue line) until the recovery specification is reached. The number of equilibrium stages is displayed.
. Stages are alternately stepped off using the equilibrium curve (red curve) and the operating line (blue line) until the recovery specification is reached. The number of equilibrium stages is displayed.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Equilibrium data was obtained from:
E. J. Henley and J. D. Seader, Equilibrium-Stage Separation Operations in Chemical Engineering, New York: Wiley, 1981.
Permanent Citation
"Absorption of Acetone in Air Using Water"
http://demonstrations.wolfram.com/AbsorptionOfAcetoneInAirUsingWater/
Wolfram Demonstrations Project
Published: March 7 2011