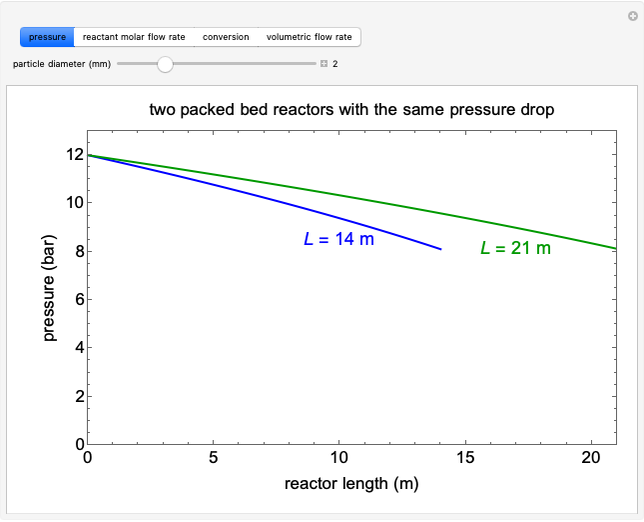
Adding a Second Component to a Single-Component Vapor-Liquid Equilibrium (VLE) Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
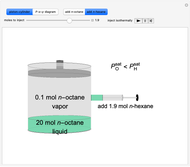
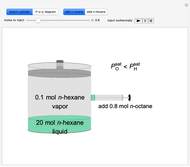
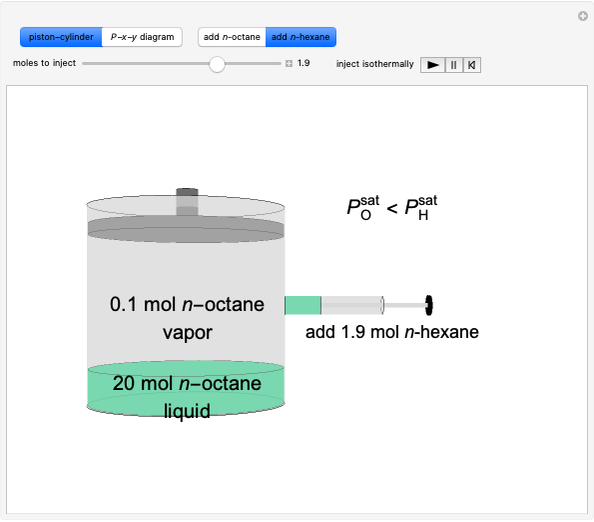
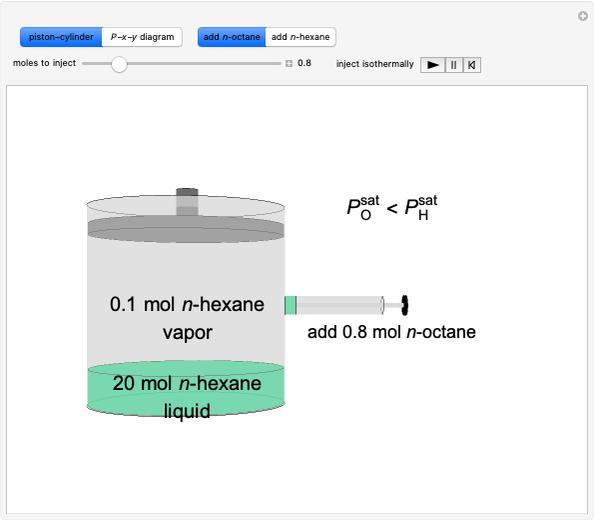
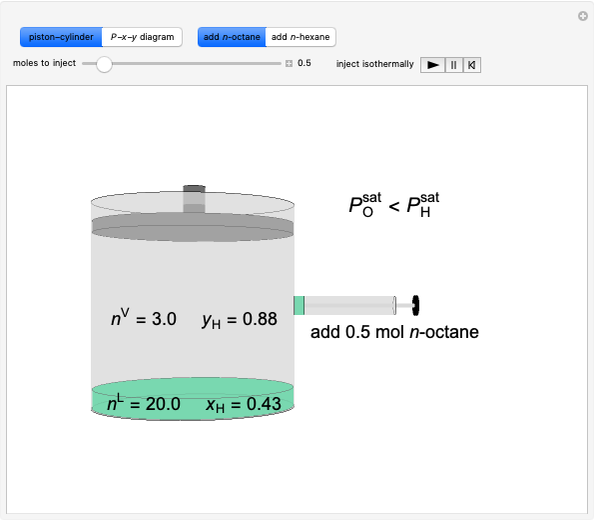
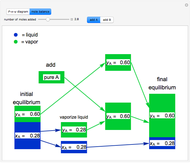
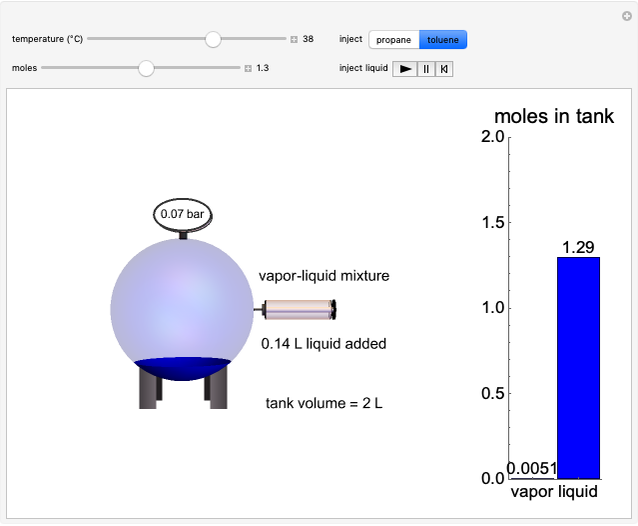
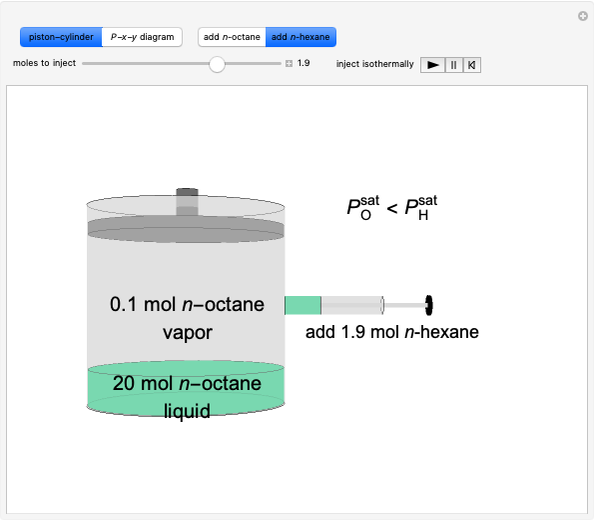
Initially, 20 mol of liquid and 0.1 mol of vapor are in equilibrium in a cylinder with a movable piston. The system initially contains only 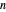 -hexane and
-hexane and 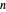 -octane is injected when the inject isothermally play button is pressed, or it starts with
-octane is injected when the inject isothermally play button is pressed, or it starts with 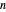 -octane and
-octane and 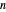 -hexane is injected. Change the number of moles injected at constant temperature and pressure with a slider. The system is modeled by Raoult’s law. The
-hexane is injected. Change the number of moles injected at constant temperature and pressure with a slider. The system is modeled by Raoult’s law. The 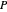 -
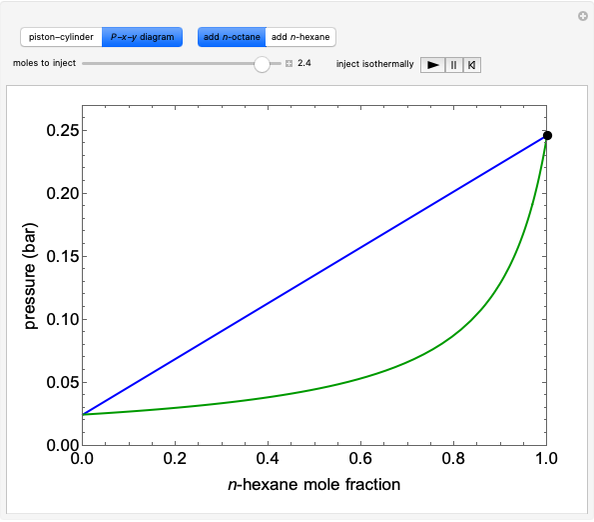
-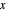 -
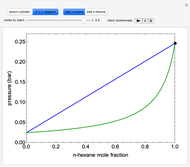
-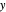 diagram explains why the final state is all liquid or all vapor. Use the reset button to start the process again.
diagram explains why the final state is all liquid or all vapor. Use the reset button to start the process again.
Contributed by: Rachael L. Baumann (August 2014)
Additional contributions by: John L. Falconer and Nick Bongiardina
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
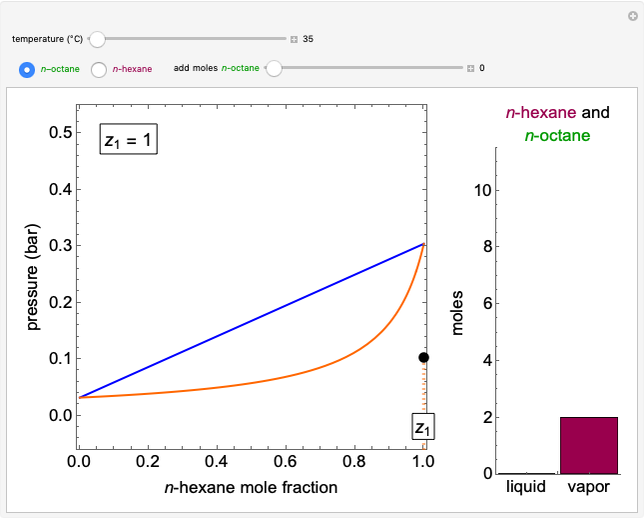
The system is modeled by Raoult's law. The 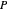 -
-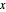 -
-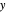 diagram shows the behavior when one component is added to the mixture.
diagram shows the behavior when one component is added to the mixture.
The saturation pressures 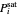 (bar) of
(bar) of 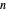 -hexane and
-hexane and 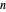 -octane are calculated using the Antoine equation, where
-octane are calculated using the Antoine equation, where 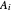 ,
, 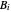 and
and 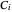 are constants,
are constants, 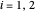 and
and 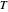 is temperature (°C):
is temperature (°C):
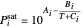 .
.
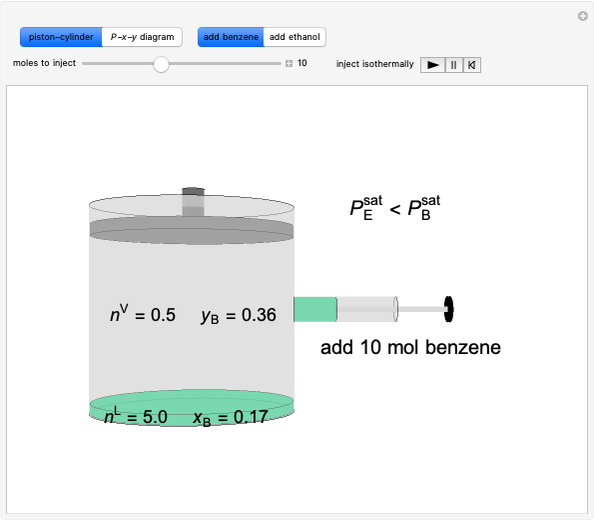
Vapor-liquid equilibrium for this ideal system exists when 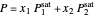 . When the system initially contains
. When the system initially contains 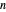 -hexane in vapor-liquid equilibrium and
-hexane in vapor-liquid equilibrium and 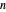 -octane (which has a lower saturation pressure) is added at constant total pressure and constant temperature, then
-octane (which has a lower saturation pressure) is added at constant total pressure and constant temperature, then 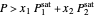 , so only liquid is present at equilibrium. When the system initially contains
, so only liquid is present at equilibrium. When the system initially contains 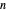 -octane in vapor-liquid equilibrium and
-octane in vapor-liquid equilibrium and 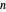 -hexane (which has a higher saturation pressure) is added at constant total pressure and constant temperature, then
-hexane (which has a higher saturation pressure) is added at constant total pressure and constant temperature, then 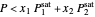 , and only vapor is present at equilibrium.
, and only vapor is present at equilibrium.
The screencast video at [1] explains how to use this Demonstration.
Reference
[1] Adding a Second Component to a Single-Component Vapor-Liquid Equilibrium (VLE) Mixture [Video]. (Dec 16, 2020) www.learncheme.com/simulations/thermodynamics/thermo-2/adding-a-second-component-to-a-single-component-vle-mixture.
Permanent Citation