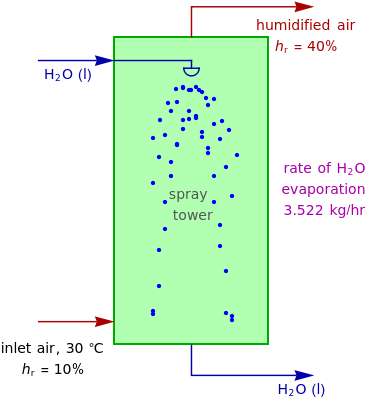
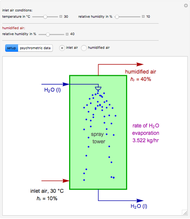
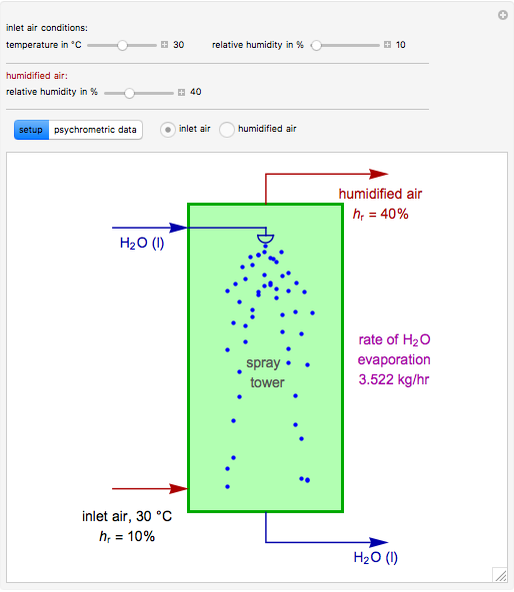
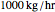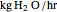In adiabatic humidification, liquid water is sprayed into a relatively dry, warm air stream in a tower. Because some water evaporates, the temperature of the air stream decreases and its moisture content increases.
Suppose that the adiabatic spray tower operates at approximately 1 atm and that the mass flow rate of the stream of air is 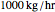 . You can set the temperature and relative humidity
. You can set the temperature and relative humidity 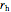 of the inlet air stream.
of the inlet air stream.
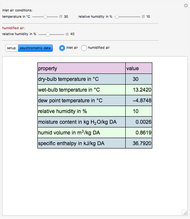
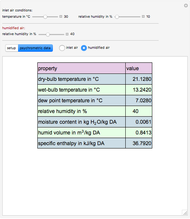
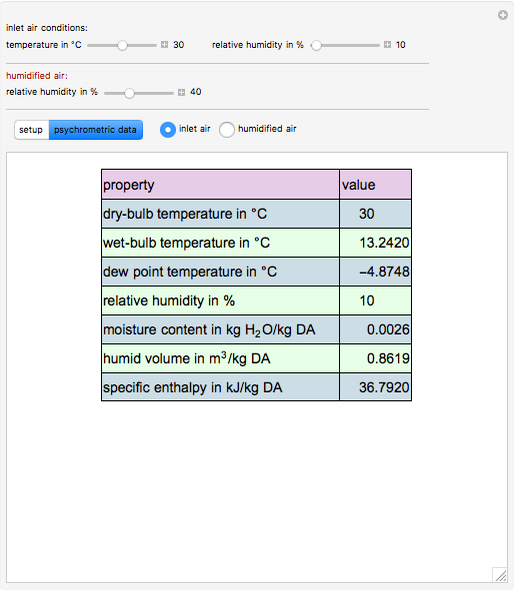
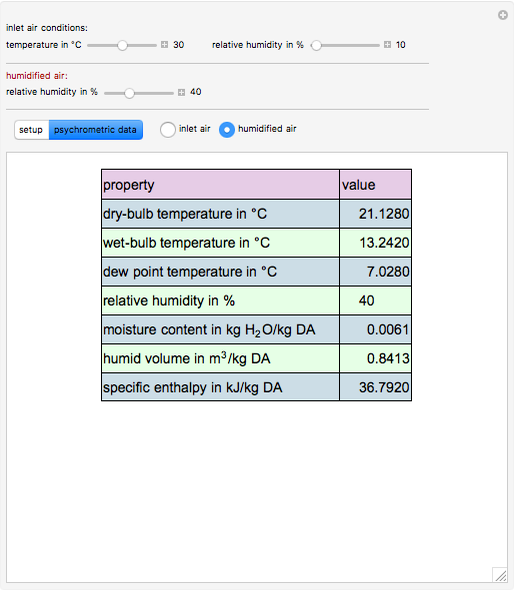
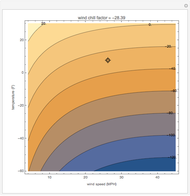
This Demonstration computes how much water must be evaporated (shown in magenta and expressed in 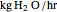 ) for a selected relative humidity of the humidified air (i.e. the outlet air stream, shown in red). In the computation, it is assumed that the rise in temperature of the water in the tower is much smaller than the heat of vaporization of water. Thus, you can follow the constant wet-bulb line on the psychrometric chart. In addition, these thermal properties of both the inlet and outlet air streams are listed:
) for a selected relative humidity of the humidified air (i.e. the outlet air stream, shown in red). In the computation, it is assumed that the rise in temperature of the water in the tower is much smaller than the heat of vaporization of water. Thus, you can follow the constant wet-bulb line on the psychrometric chart. In addition, these thermal properties of both the inlet and outlet air streams are listed:
1. the dry-bulb temperature
2. the wet-bulb temperature
3. the dew point temperature
4. the relative humidity
5. the absolute humidity or moisture content
6. the humid volume
7. the specific enthalpy
Things to try out or check:
1. For fixed temperature and relative humidity of the inlet air stream, increase the relative humidity of the humidified air stream. As expected, you will observe an increase in the computed amount of added water.
2. The values of the wet-bulb temperature and specific heat of both the inlet and outlet air streams are identical. This is expected, because we assume adiabatic humidification that moves along a constant wet-bulb line on the psychrometric chart.
3. The dry-bulb temperature of the humidified air is, as expected, lower than the temperature of the inlet air.
[less]