Arrhenius Equation for Chemical Reaction

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
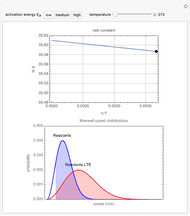
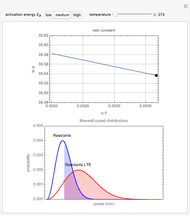
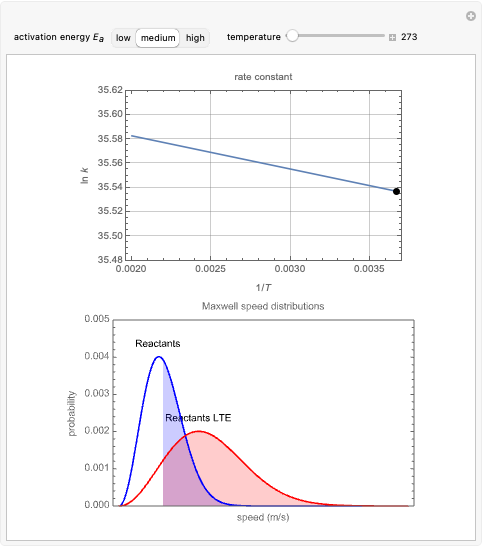
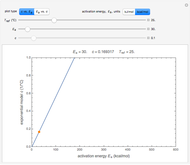
The plots show examples of the Arrhenius equation 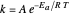 for variations of the rate constant
for variations of the rate constant  with temperature
with temperature 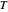 and activation energy
and activation energy 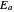 for a typical chemical reaction;
for a typical chemical reaction; 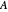 is a constant and
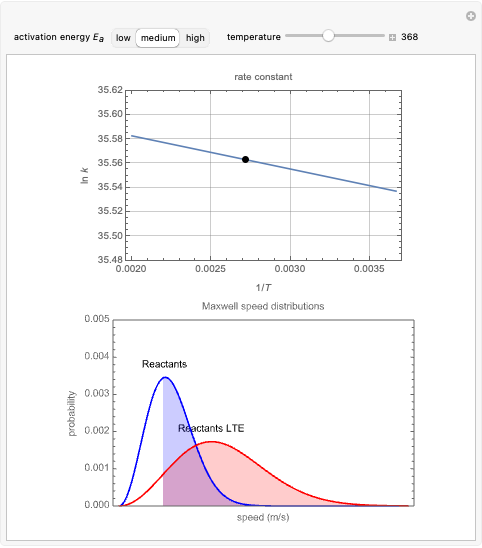
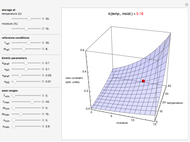
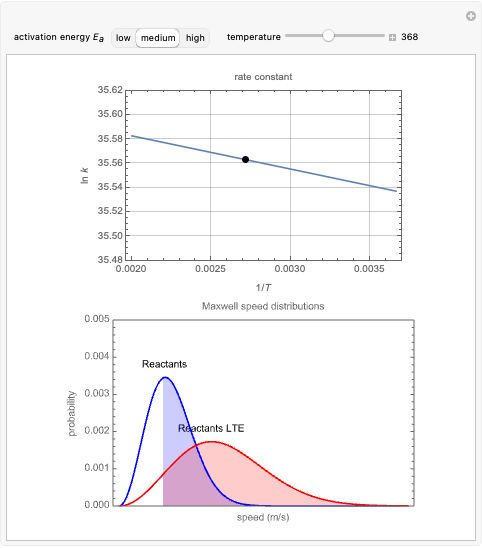
is a constant and 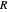 is the universal gas constant. This explains how reactions are generally faster at higher temperatures. The lower plot depicts Maxwell speed distributions of molecules showing the fractions of molecules with sufficient energy to overcome the activation energy barrier, as a function of temperature. The shaded regions represent the
is the universal gas constant. This explains how reactions are generally faster at higher temperatures. The lower plot depicts Maxwell speed distributions of molecules showing the fractions of molecules with sufficient energy to overcome the activation energy barrier, as a function of temperature. The shaded regions represent the 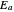 values, which enable the reaction to proceed. These can be changed with the "activation energy" buttons.
values, which enable the reaction to proceed. These can be changed with the "activation energy" buttons.
Contributed by: Natalie Neumann and Harshil Bhavsar (August 25)
Advised by: Heidi Hendrickson
Open content licensed under CC BY-NC-SA
Details
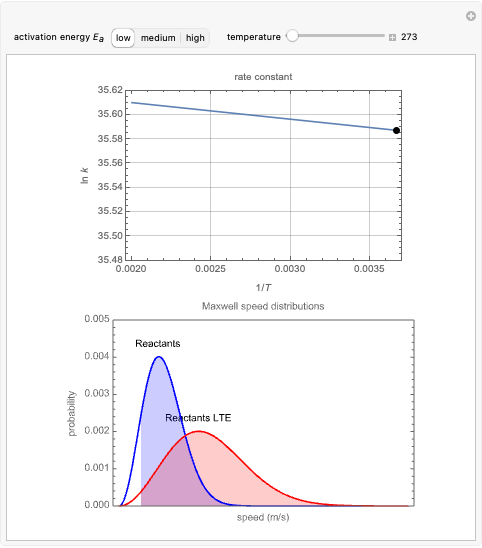
Snapshot 1: In the top plot, alterations in temperature affect the rate constant  at a low activation energy. In the bottom plot, the fraction of molecules in a generic reactants-to-products reaction that have enough energy to overcome the activation energy at user-defined temperatures appears as shaded regions.
at a low activation energy. In the bottom plot, the fraction of molecules in a generic reactants-to-products reaction that have enough energy to overcome the activation energy at user-defined temperatures appears as shaded regions.
Snapshots 2 and 3: The same as snapshot 1, but now showing how temperature affects  and
and  at medium and high activation energies. All snapshots are shown at 273 K.
at medium and high activation energies. All snapshots are shown at 273 K.
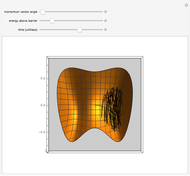
The Maxwell speed distribution is used to model how a generic reaction is affected by temperatures of the reactant and product molecules. This distribution for gas-phase particles is modeled after kinetics of the aqueous decomposition of a benzenediazonium (BDA) derivative from aniline. In order to display adequate kinetics in the Demonstration, energy values were taken from [1] so that a real process could be modeled by the Maxwell speed distribution. This graph also includes a comparison to one-fourth of the molar mass of a regular BDA molecule.
Activation energy values and the decomposition reaction were derived from [1] for realistic kinetic parameters.
Reference
[1] M. L. Crossley, R. H. Kienle and C. H. Benbrook, "Chemical Constitution and Reactivity. I. Phenyldiazonium Chloride and Its Mono Substituted Derivatives," Journal of the American Chemical Society, 62(6), 1940 pp. 1400–1404. doi:10.1021/ja01863a019.
Snapshots
Permanent Citation