Boiling Point Elevation of Solutions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
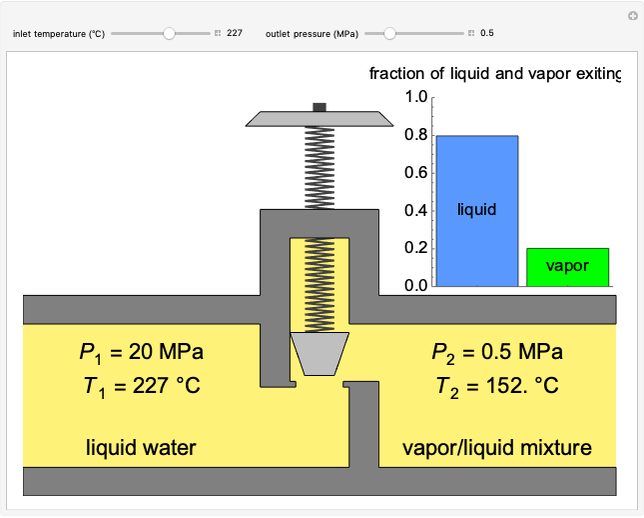
When a solute is added to a solvent, the boiling point of the resulting solution increases, according to the equation 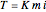 , where
, where 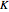 is the ebullioscopic constant (specific to each solvent),
is the ebullioscopic constant (specific to each solvent), 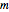 is the molality (moles solute over kilograms solvent), and
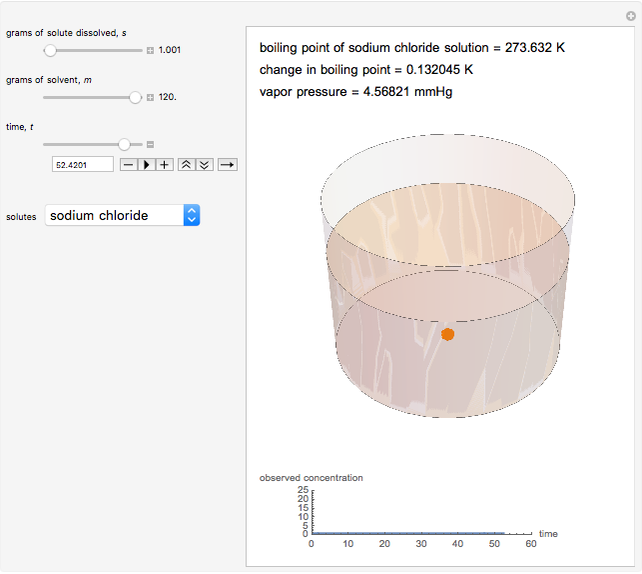
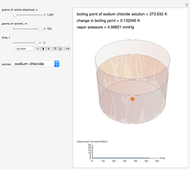
is the molality (moles solute over kilograms solvent), and 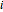 is the van't Hoff factor, which depends on the extent to which the solute ionizes in solution. A solution boils when the vapor pressure is equal to the atmospheric pressure. When adding a solute to a solvent, the vapor pressure of the resulting solution is lower than the vapor pressure of the original solvent, so it takes a higher temperature for the vapor pressure to equal atmospheric pressure, ultimately resulting in an elevated boiling point. This Demonstration shows the boiling point elevation from solute and solvent data. A graphic approximating the progress of the dissolution process with time is also shown.
is the van't Hoff factor, which depends on the extent to which the solute ionizes in solution. A solution boils when the vapor pressure is equal to the atmospheric pressure. When adding a solute to a solvent, the vapor pressure of the resulting solution is lower than the vapor pressure of the original solvent, so it takes a higher temperature for the vapor pressure to equal atmospheric pressure, ultimately resulting in an elevated boiling point. This Demonstration shows the boiling point elevation from solute and solvent data. A graphic approximating the progress of the dissolution process with time is also shown.
Contributed by: Ramish Zaidi and Vignesh Karthikeyan (June 2015)
Based on a program by: Enrique Zeleny
With additional contributions by: Christopher Grattoni
Special thanks to the University of Illinois NetMath program and the mathematics department at William Fremd High School.
Open content licensed under CC BY-NC-SA
Snapshots
Details
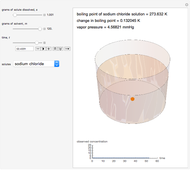
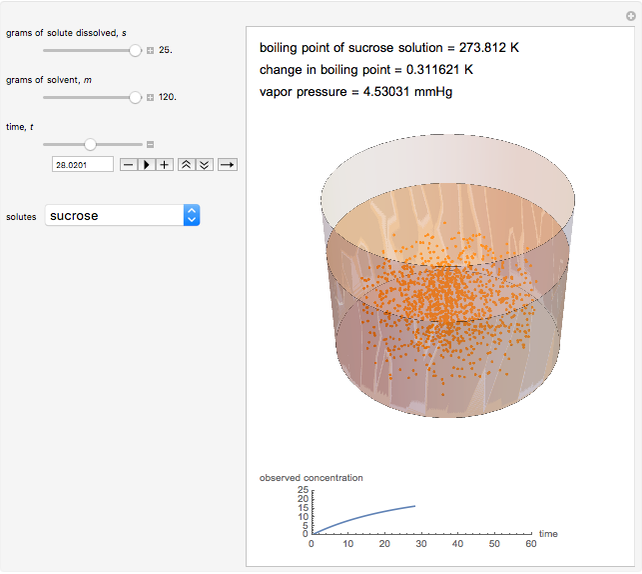
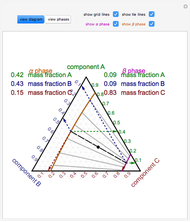
Snapshot 1: This shows how little solute dissolves when it has a mass much lower than that of the solvent; the subsequent change in boiling point is small.
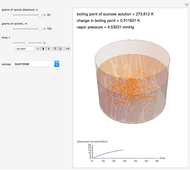
Snapshot 2: This shows how the graphic looks with maximum amounts of solute and solvent; the boiling point is higher than that in the preceding snapshot. The graph at the bottom right has changed, reflecting the chemistry.
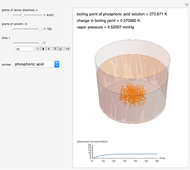
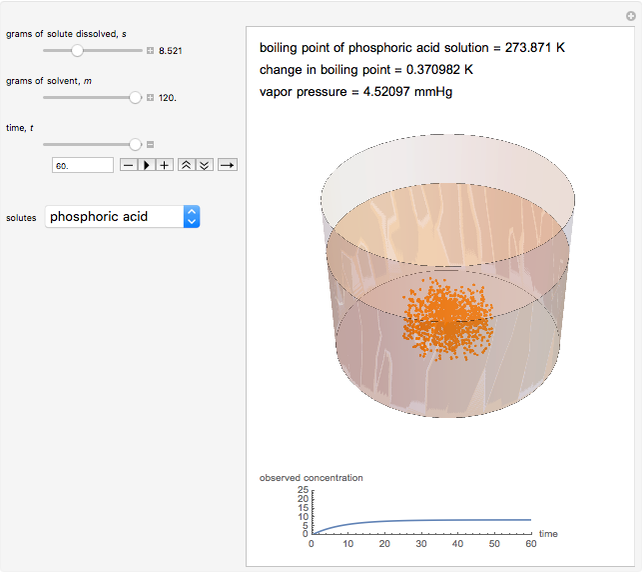
Snapshot 3: This shows an intermediate amount of solute; the solute type has been changed. An intermediate amount of phosphoric acid has more of an effect on boiling point than the maximum amounts of sucrose. The time has been maximized to observe the changes in the graph.
References
[1] The ChemTeam. "Boiling Point Elevation." (Jun 8, 2015) www.chemteam.info/Solutions/BP-elevation.html.
[2] UC Davis ChemWiki. "Vapor Pressure." (Jun 8, 2015) chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_of _Matter/Phases_of_Matter/Liquids/Vapor_Pressure.
Permanent Citation
"Boiling Point Elevation of Solutions"
http://demonstrations.wolfram.com/BoilingPointElevationOfSolutions/
Wolfram Demonstrations Project
Published: June 9 2015