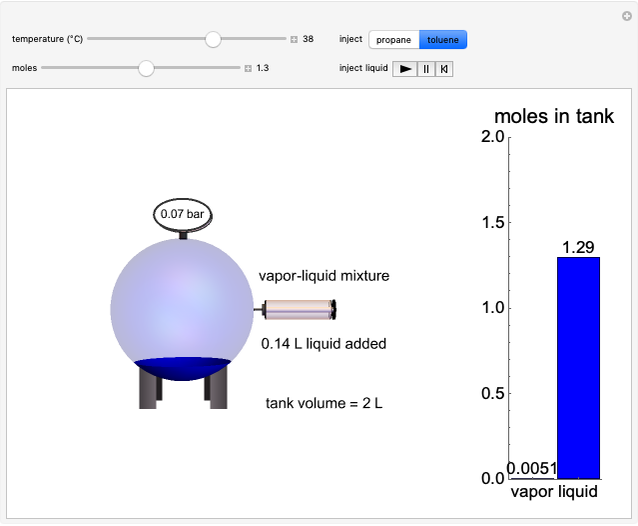
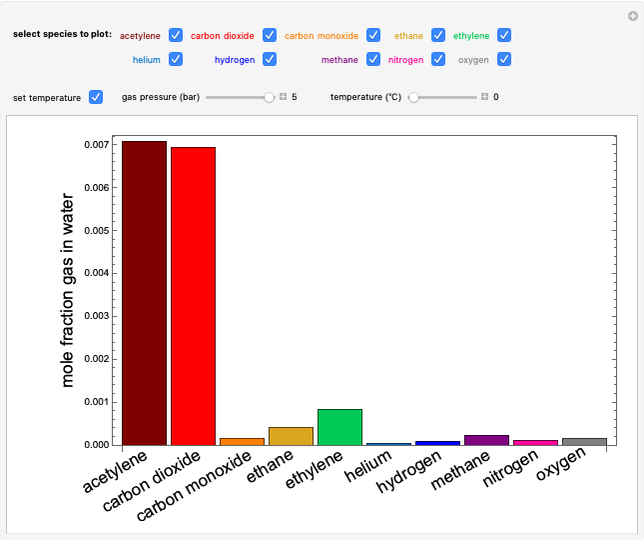
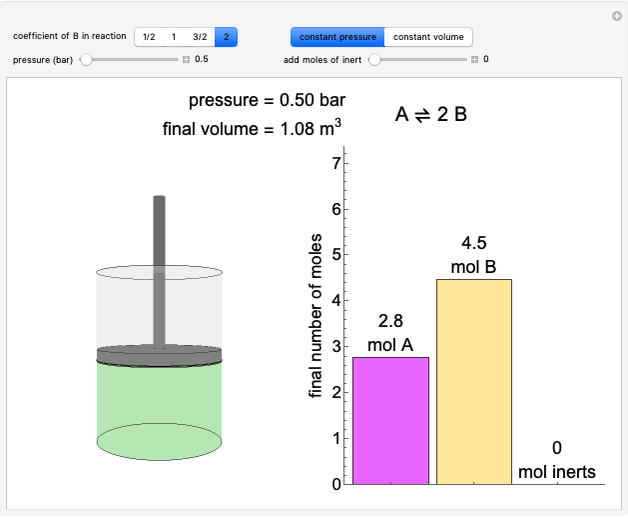
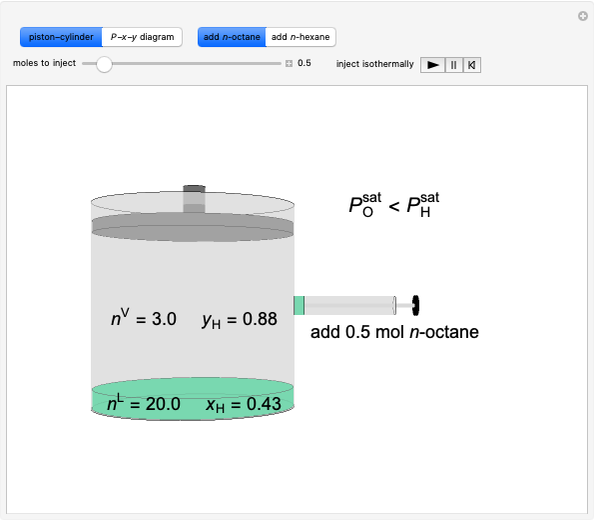
Construct an x-y Diagram for a Stripping Column

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
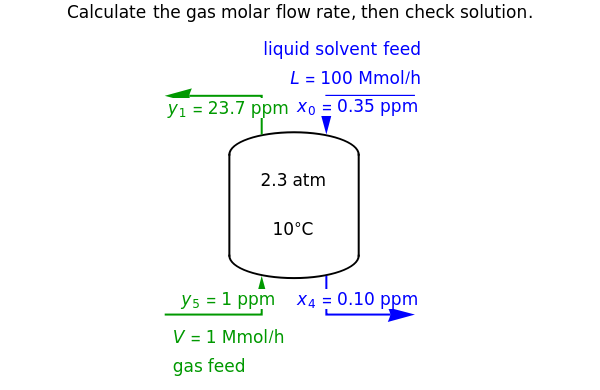
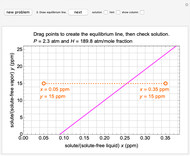
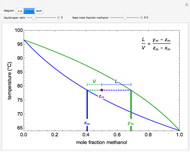
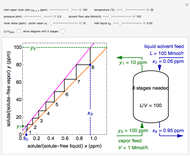
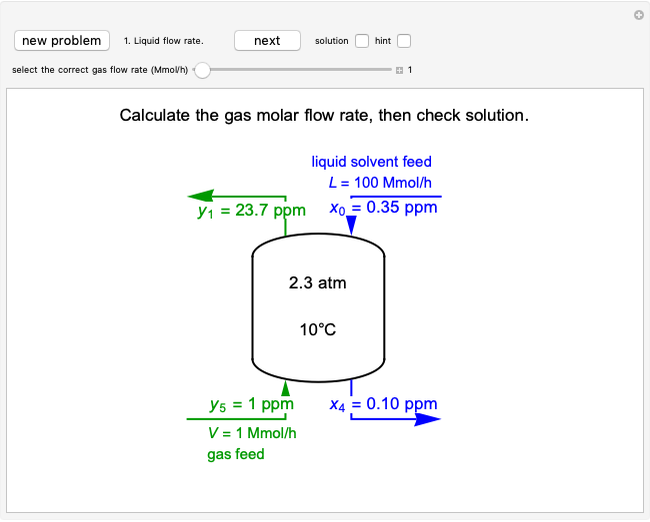
In this Demonstration, a trayed stripping column is used to remove an impurity from a liquid feed by stripping the impurity into a gas stream. A stage is a plate that contacts the liquid solvent and the gas to promote mass transfer. Construct an  -
- diagram step by step to determine the number of stages needed to achieve a certain separation. The operating line is obtained from a mass balance, and the phase equilibrium line is obtained from Henry's law. At any time, click the "new problem" button to generate a new set of conditions. Check "solution" to see the answer before clicking "next" to move on to the next step. Once you check "solution," you cannot change your answer. Once you click "next," you cannot go back to the previous steps. Check "hint" for help at any stage.
diagram step by step to determine the number of stages needed to achieve a certain separation. The operating line is obtained from a mass balance, and the phase equilibrium line is obtained from Henry's law. At any time, click the "new problem" button to generate a new set of conditions. Check "solution" to see the answer before clicking "next" to move on to the next step. Once you check "solution," you cannot change your answer. Once you click "next," you cannot go back to the previous steps. Check "hint" for help at any stage.
Contributed by: Rachael L. Baumann (May 2018)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
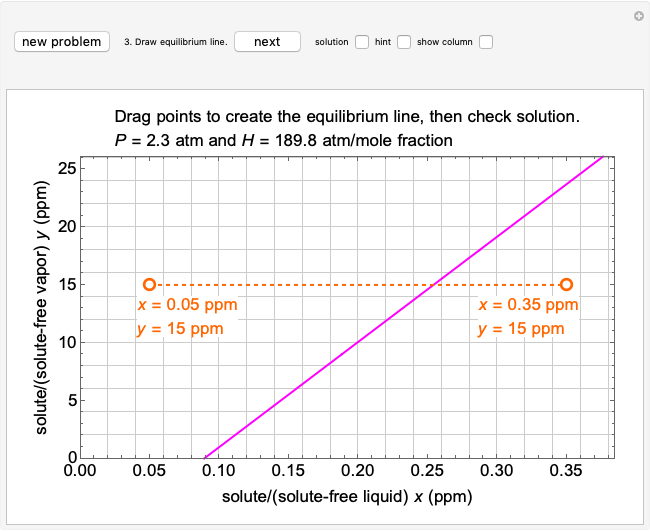
The equilibrium line is calculated using Henry's law:
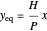 ,
,
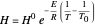 ,
,
where 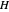 is Henry's constant (atm),
is Henry's constant (atm), 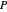 is pressure (atm),
is pressure (atm), 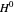 is Henry's constant at
is Henry's constant at 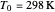 (atm),
(atm), 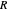 is the ideal gas constant (J/mol K), and
is the ideal gas constant (J/mol K), and 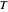 is temperature (K).
is temperature (K).
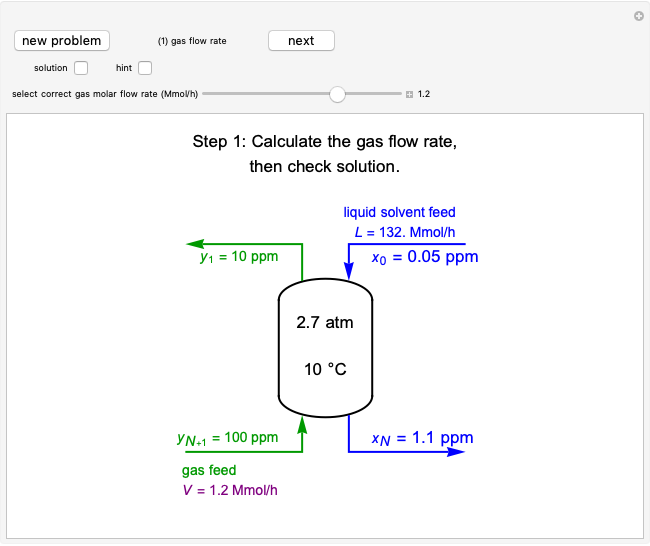
The operating line is calculated from a mass balance around the stripper:
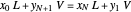 ,
,
which rearranges to:
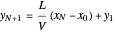 .
.
The operating line is:
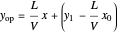 ,
,
where 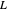 is the liquid solvent molar flow rate (Mmol/h),
is the liquid solvent molar flow rate (Mmol/h), 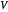 is the vapor molar flow rate (Mmol/h),
is the vapor molar flow rate (Mmol/h), 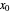 is the mole ratio of the impurity in the inlet liquid solvent stream (ppm),
is the mole ratio of the impurity in the inlet liquid solvent stream (ppm), 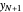 is the mole ratio of the impurity in the inlet vapor stream (ppm),
is the mole ratio of the impurity in the inlet vapor stream (ppm), 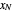 is the mole ratio of the impurity in the outlet liquid solvent stream (ppm) and
is the mole ratio of the impurity in the outlet liquid solvent stream (ppm) and 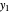 is the mole ratio of the impurity in the outlet vapor stream (ppm).
is the mole ratio of the impurity in the outlet vapor stream (ppm).
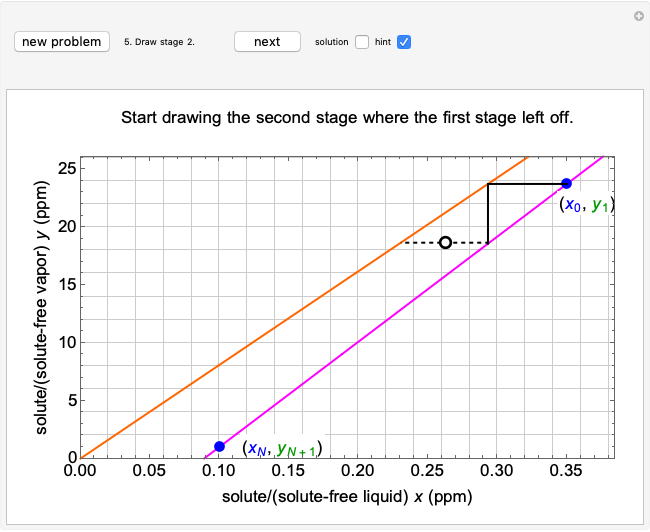
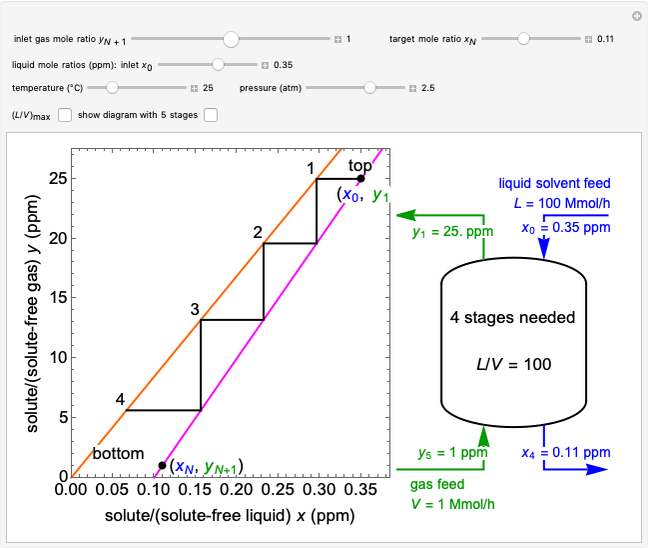
To count off stages, start at 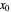 on the operating line (
on the operating line (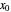 ,
, 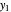 ) and draw a horizontal line to the equilibrium line
) and draw a horizontal line to the equilibrium line 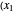 ,
, 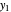 ). Then draw a vertical line down to the operating line. Repeat these steps until
). Then draw a vertical line down to the operating line. Repeat these steps until 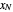 is reached.
is reached.
The outlet liquid mole ratio 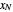 is calculated from the mass balance:
is calculated from the mass balance:
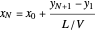 .
.
Reference
[1] P. C. Wankat, Separation Process Engineering: Includes Mass Transfer Analysis, 3rd ed., Upper Saddle River, NJ: Prentice Hall, 2012.
Permanent Citation