Converting Potentials between Different Reference Electrodes

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
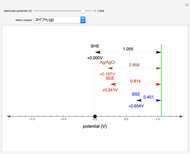
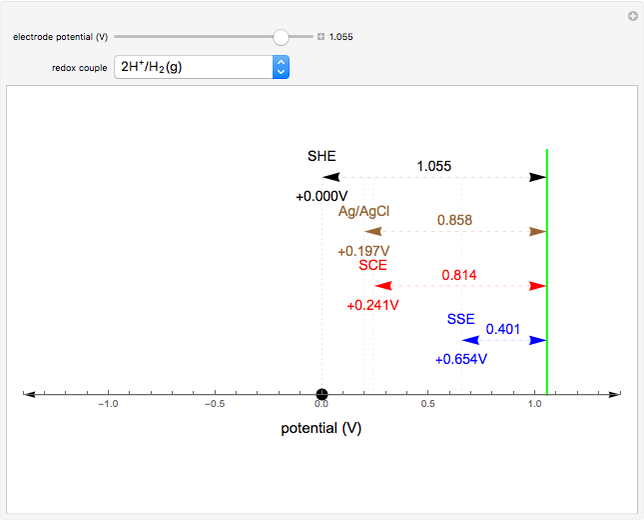
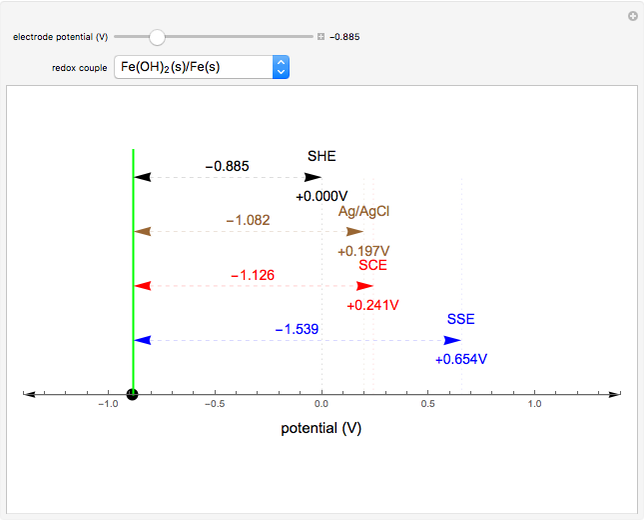
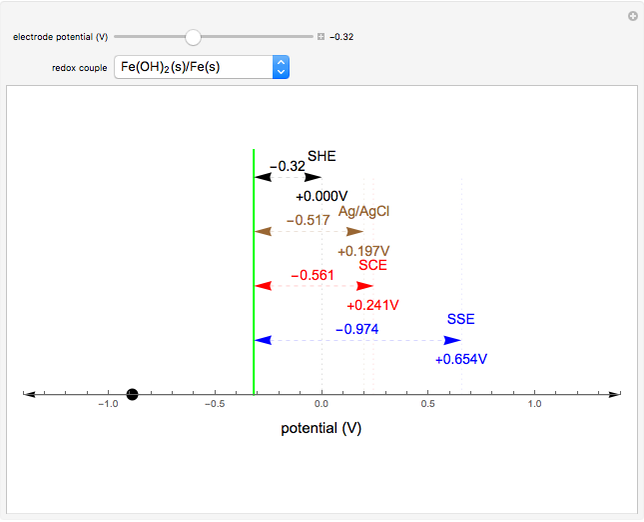
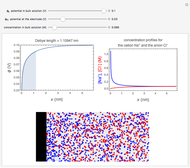
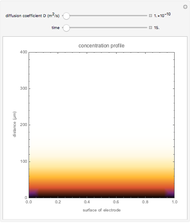
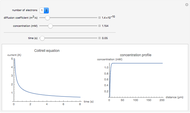
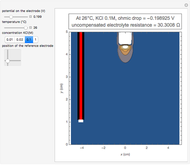
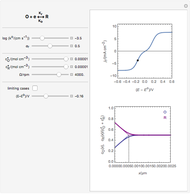
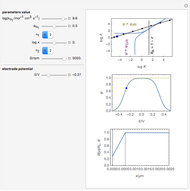
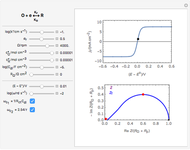
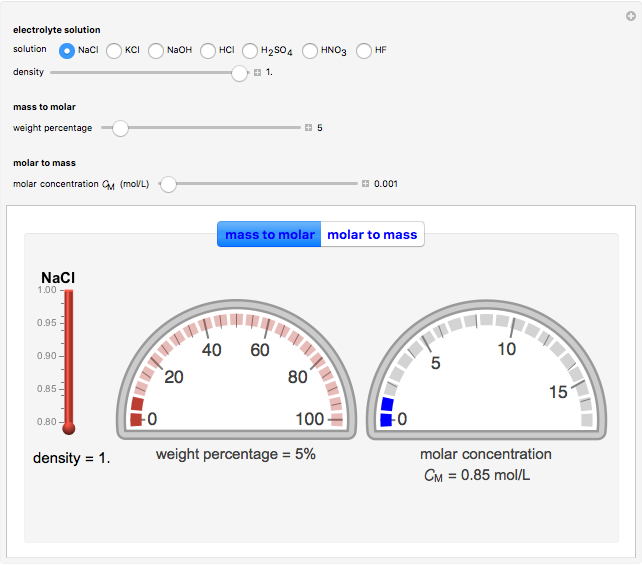
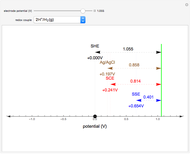
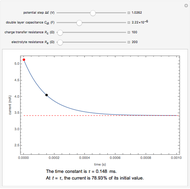
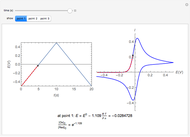
Many different reference electrodes can be used in electrochemical experiments. We consider the following four: standard hydrogen electrode (SHE), Ag/AgCl reference electrode, saturated calomel electrode (SCE), and saturated sulfate electrode (SSE). This Demonstration converts the electrode potential between different reference electrodes. The standard electrode potentials of some common redox couples are also provided. Slide the electrode potential (shown as a green vertical line) to convert it to the desired reference electrode or to compare this electrode potential to the standard electrode potential of the chosen redox couple (presented as a black point on the potential axis).
Contributed by: Quang-Dao Trinh (May 2016)
Open content licensed under CC BY-NC-SA
Snapshots
Details
A reference electrode must have a stable and well-defined potential. Standard electrode potentials are reported with reference to the SHE. However, the SHE is difficult to use in practice as it involves bubbling hydrogen gas. Other reference electrodes that are widely used are SCE, SSE, and Ag/AgCl.
The SCE is the most commonly used reference electrode. However, it contains mercury, so this electrode cannot be used above 50°C due to the instability of 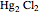 .
.
The Ag/AgCl reference electrode is simpler in construction than the SCE and it contains no mercury.
The SSE reference electrode is recommended when the chloride ion cannot be tolerated in the electrolyte.