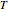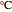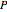Converting Syngas to Ethanol
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
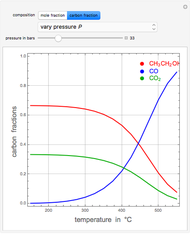
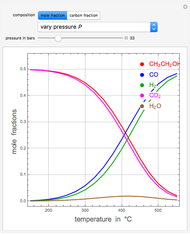
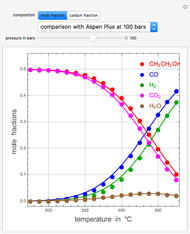
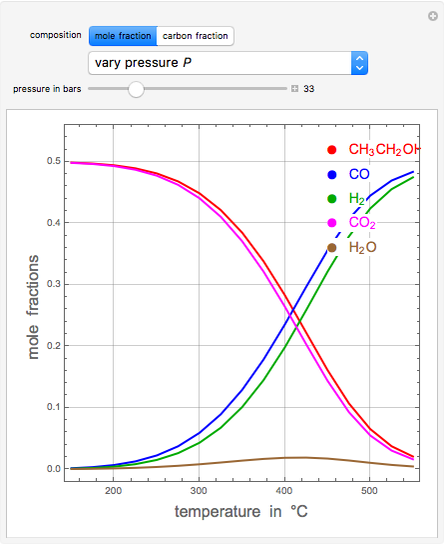
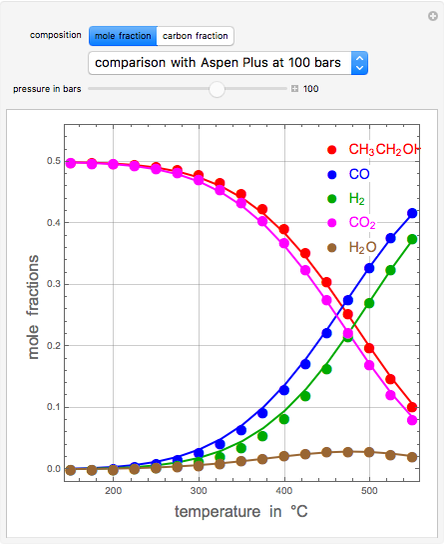
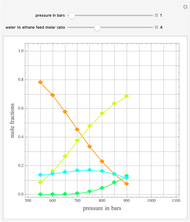
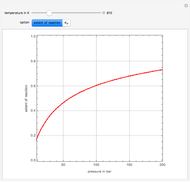
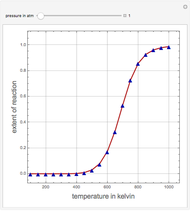
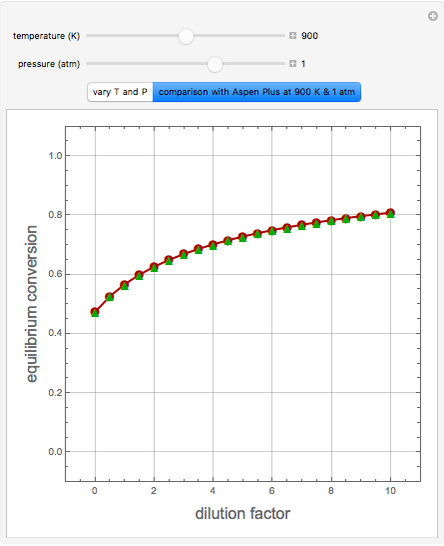
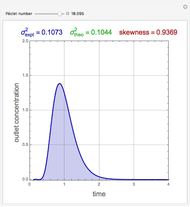
A thermodynamic analysis is essential to understanding the equilibrium limits in converting syngas to ethanol. This Demonstration considers these five chemical species: 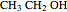 (ethanol),
(ethanol), 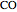 and
and 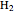 (syngas),
(syngas), 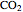 , and
, and 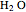 . The feed composition is such that the molar ratio
. The feed composition is such that the molar ratio 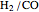 is 1.
is 1.
Contributed by: Housam Binous, Mohammad Mozahar Hossain, and Ahmed Bellagi (November 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
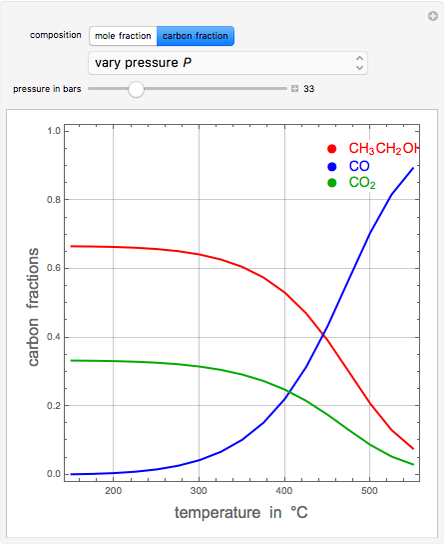
Let 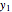 ,
, 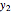 , and
, and 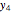 be the mole fractions of
be the mole fractions of 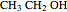 (ethanol),
(ethanol), 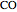 , and
, and 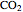 , respectively. Then the carbon fractions are given by:
, respectively. Then the carbon fractions are given by:
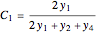 (for ethanol),
(for ethanol), 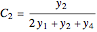 (for
(for 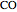 ), and
), and 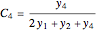 (for
(for 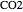 ).
).
Permanent Citation