Corrosion Mechanism for Stainless Steel

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
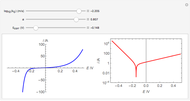
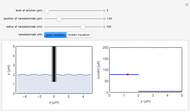
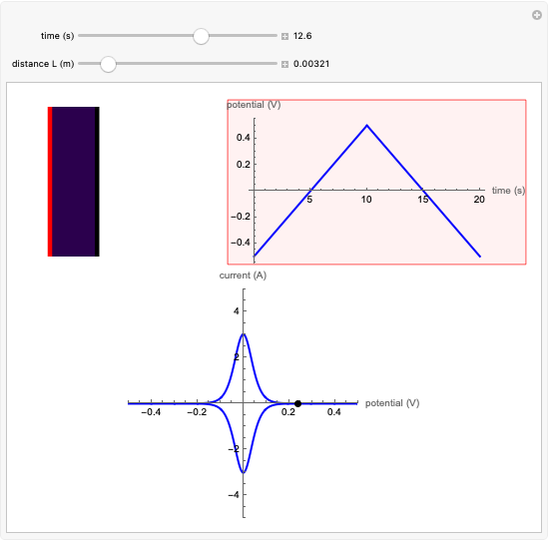
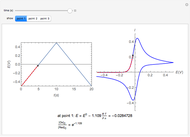
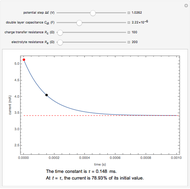
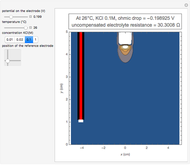
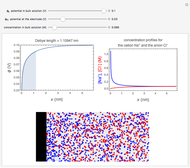
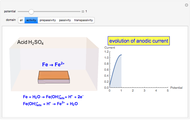
Corrosion degrades materials, which is a source of significant expense. This Demonstration explains how steel corrodes. A steel plate (whose main component is iron) is corroded in an acid solution. This plate plays the role of a working electrode in an electrochemical cell, for which the current and potential can be measured.
Contributed by: Quang-Dao Trinh (December 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
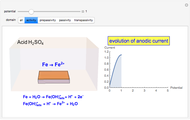
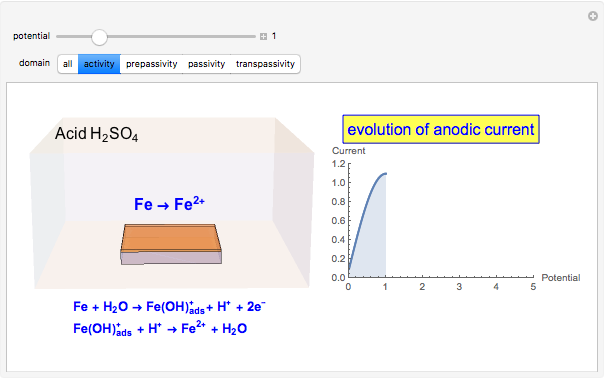
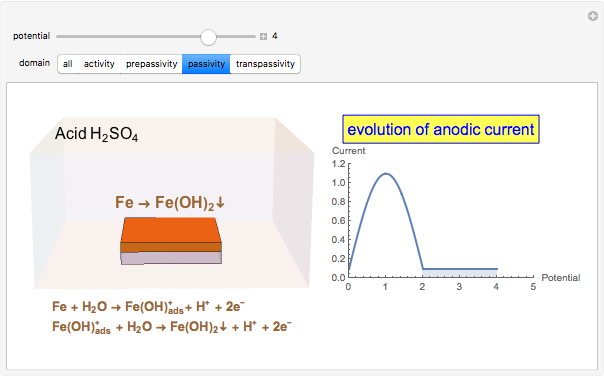
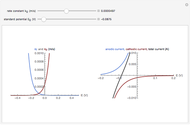
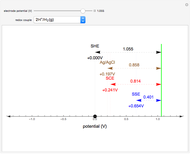
When the metal is dissolved in a corrosive environment, the anodic reaction takes place at the interface.
In the activity domain, the anodic current increases and the overall reaction is 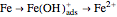 .
.
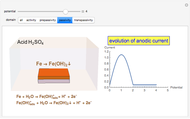
In the prepassivity domain, the formation of the oxide film 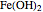 protects the metal from the corrosive environment. The anodic current decreases according to the reaction
protects the metal from the corrosive environment. The anodic current decreases according to the reaction
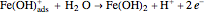 .
.
In the passivity domain, the protective layer totally separates the metal from the environment. The anodic current is stable and at a minimum.
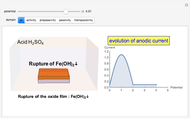
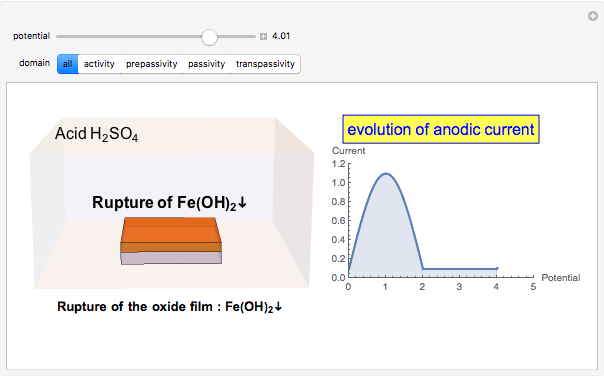
In the transpassivity domain, the layer ruptures. The metal continues to corrode and the anodic current again increases via
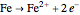 .
.
Permanent Citation