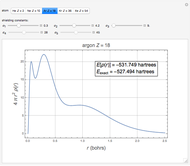
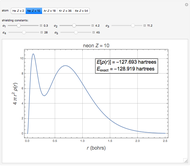
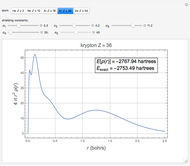
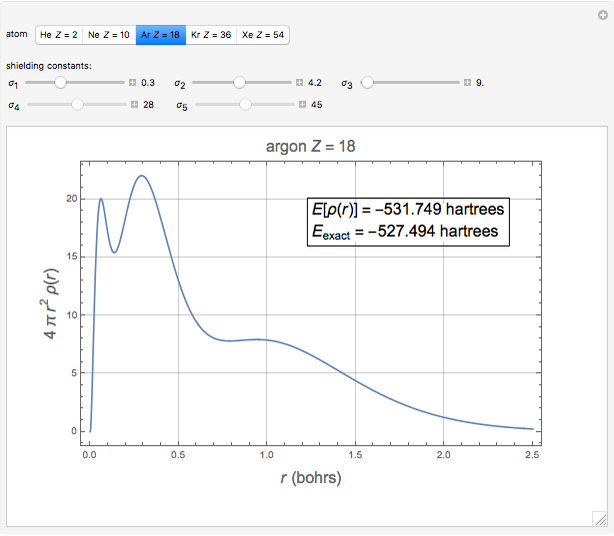
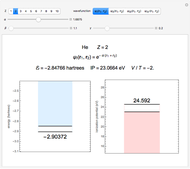
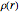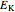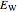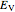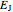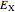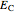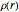Density Functional Computations on Noble Gas Atoms

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
Density functional theory (DFT) has now become the predominant technique in computational quantum chemistry, having displaced wavefunction-based computations for atoms, molecules and solids. The key reason is that QFT deals with a single electron density function 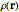 for an
for an  -electron system, rather than a complicated combination of
-electron system, rather than a complicated combination of  orbital functions
orbital functions 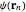 . The fundamental validity of DFT and its practical implementation by a variational principle are expressed in two theorems of Hohenberg and Kohn. For all necessary background on DFT, refer to the definitive monograph of Parr and Yang [1]. For more recent advances, see also [2].
. The fundamental validity of DFT and its practical implementation by a variational principle are expressed in two theorems of Hohenberg and Kohn. For all necessary background on DFT, refer to the definitive monograph of Parr and Yang [1]. For more recent advances, see also [2].
Contributed by: S. M. Blinder (June 2019)
Open content licensed under CC BY-NC-SA
Details
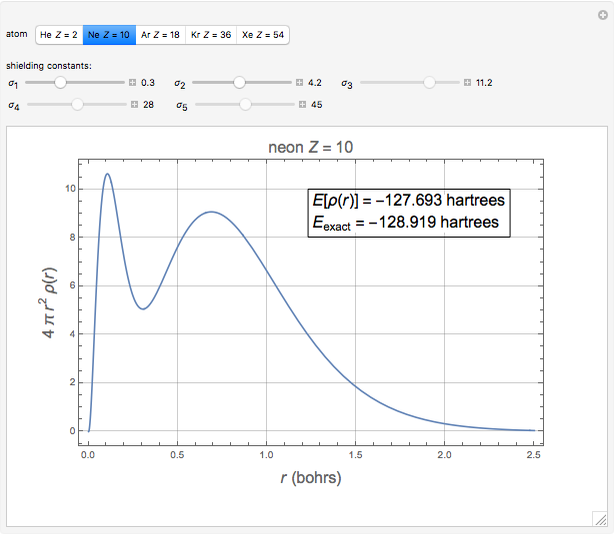
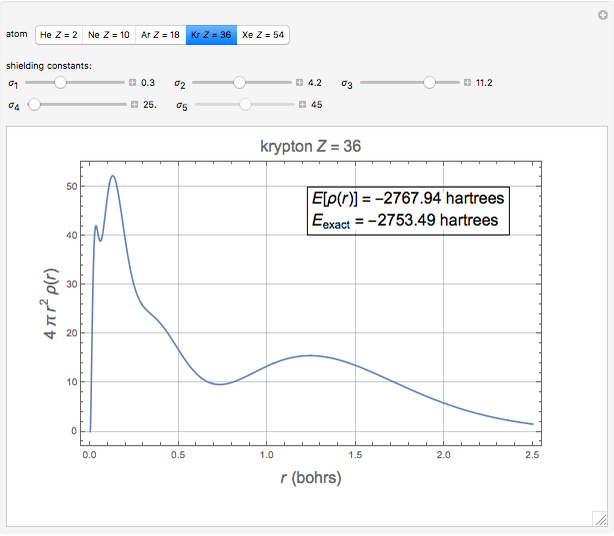
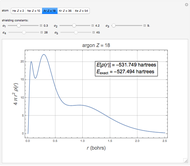
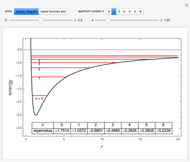
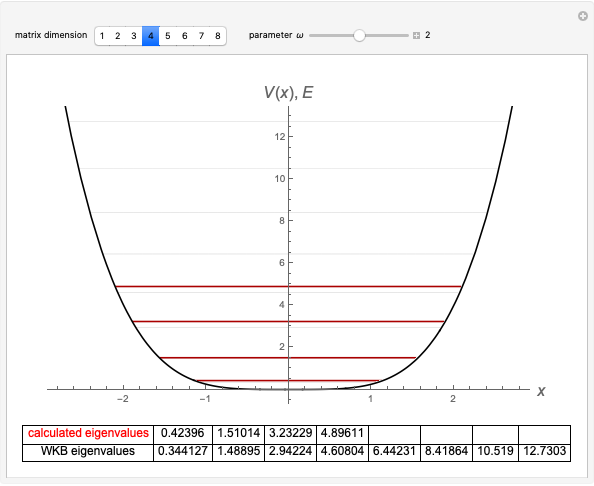
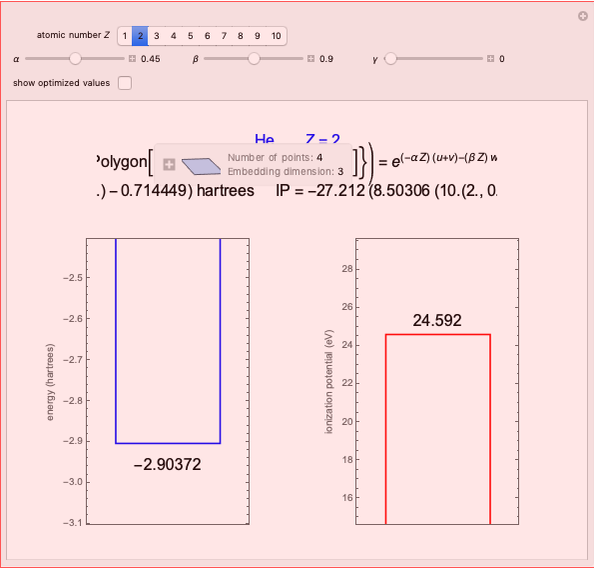
The total electron density is approximated by a sum of shells (one to five shells for He to Xe):
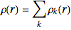 , where
, where 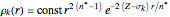 ,
,
which is suggested by Slater's rules for atomic orbitals.
The DFT functional takes the form
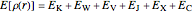 ,
,
with
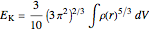 ,
,
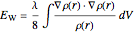 ,
, 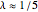 ,
,
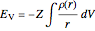 ,
,
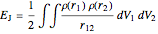 ,
,
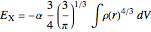 ,
, 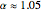 ,
,
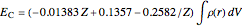 .
.
The last formula is a conjecture by the author based on computations of atomic correlation energies.
It is most convenient to carry out all the integrals numerically. The energy functional 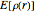 , based on the selected shielding parameters
, based on the selected shielding parameters 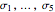 , is computed and compared with the exact (nonrelativistic) energy of the atom. By the second Hohenberg–Kohn theorem, the optimized energy for the functional form of
, is computed and compared with the exact (nonrelativistic) energy of the atom. By the second Hohenberg–Kohn theorem, the optimized energy for the functional form of 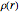 is a minimum, although short of the exact energy.
is a minimum, although short of the exact energy.
References
[1] R. G. Parr and W. Yang, Density-Functional Theory of Atoms and Molecules, New York: Oxford University Press, 1989.
[2] J. Sun, J. W. Furness and Y. Zhang, "Density Functional Theory," Mathematical Physics in Theoretical Chemistry (S. M. Blinder and J. E. House, eds.), Amsterdam: Elsevier, 2018 Chapter 4. doi:10.1016/B978-0-12-813651-5.00004-8.
[3] W.-P. Wang and R. G. Parr, "Statistical Atomic Models with Piecewise Exponentially Decaying Electron Densities," Physical Review A, 16(3), 1977 pp. 891–902. doi:10.1103/PhysRevA.16.891.
[4] S. M. Blinder. "Shell Structure of Noble Gas Atoms" from the Wolfram Demonstrations Project—A Wolfram Web Resource. demonstrations.wolfram.com/ShellStructureOfNobleGasAtoms.
Snapshots
Permanent Citation