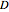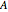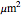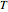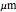Diffusion of Oxygen Molecules across a Membrane Using Fick's Law

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
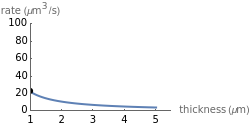
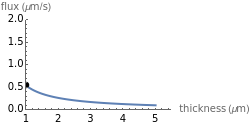
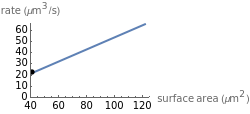
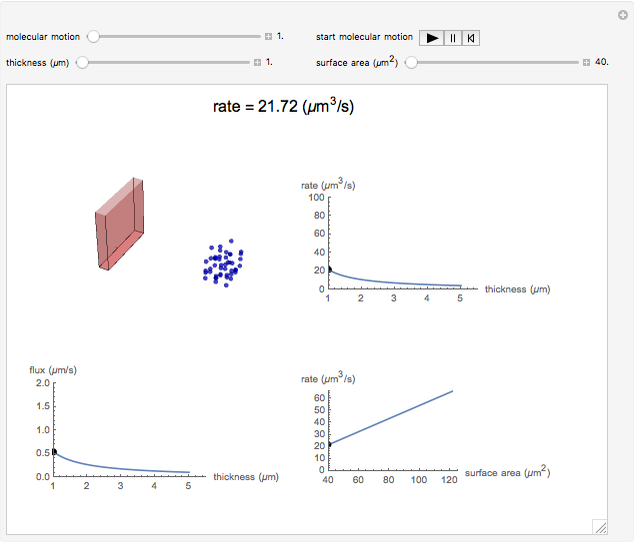
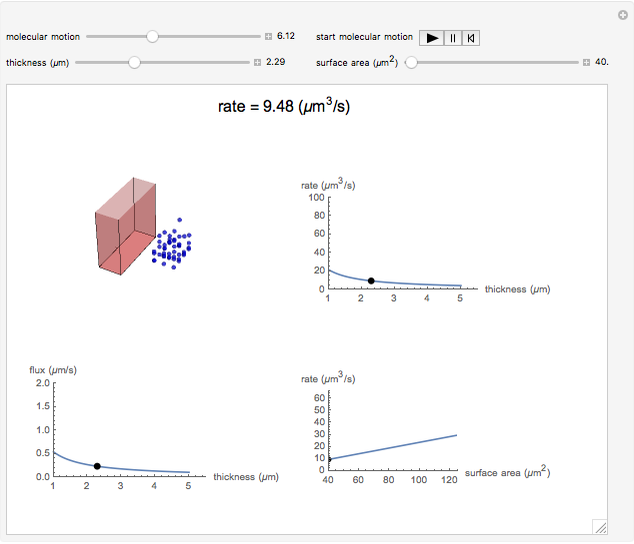
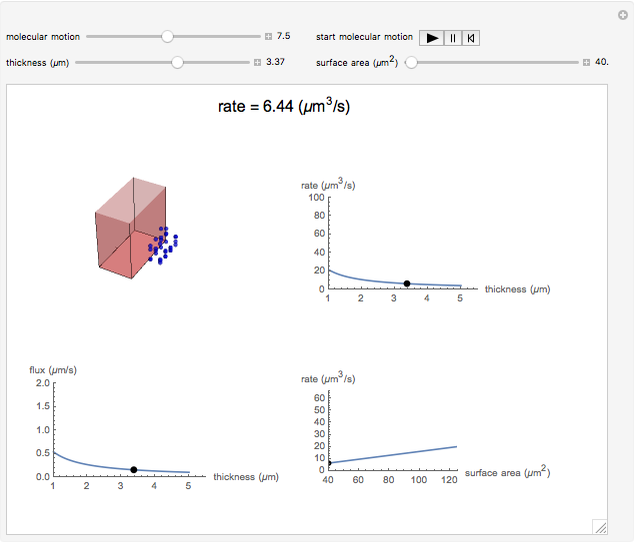
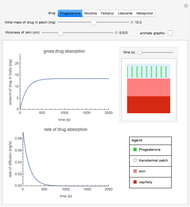
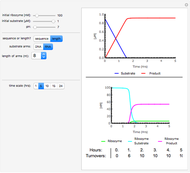
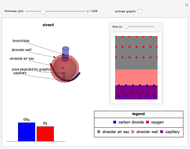
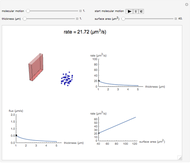
This Demonstration shows the diffusion of oxygen molecules across a membrane (represented by a box) according to Fick’s law. The graphic is not to scale and the molecular motion is idealized.
[more]
Contributed by: Neha Paruchuri and Carla Obeid (April 2017)
Additional contributions by: Eitan Geva (University of Michigan)
Open content licensed under CC BY-NC-SA
Details
References
[1] T. K. Goldstick, V. T. Ciuryla and L. Zuckerman, "Diffusion of Oxygen in Plasma and Blood," Advances in Experimental Medicine and Biology, 75, 1976 pp. 183–190. www.ncbi.nlm.nih.gov/pubmed/1015403.
[2] Pathway Medicine. "Diffusing Capacity." (Mar 27, 2017) www.pathwaymedicine.org/diffusing-capacity.
Submission from the Compute-to-Learn course at the University of Michigan.
Snapshots
Permanent Citation