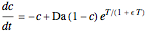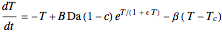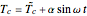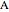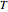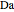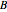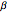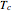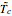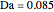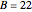Dynamics of a Forced Exothermic Chemical Reaction
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
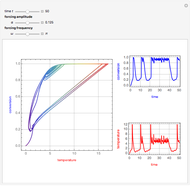
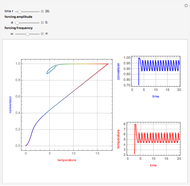
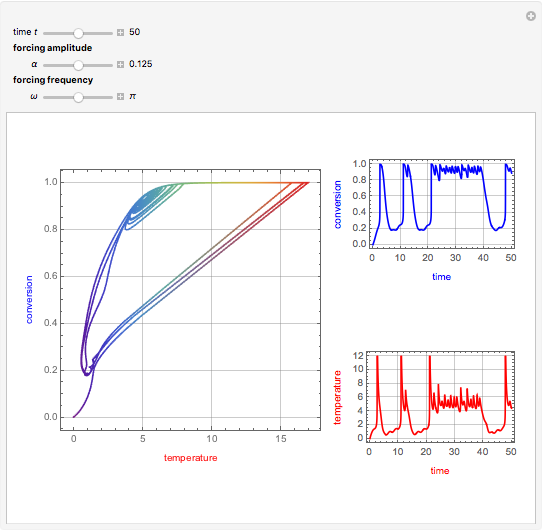
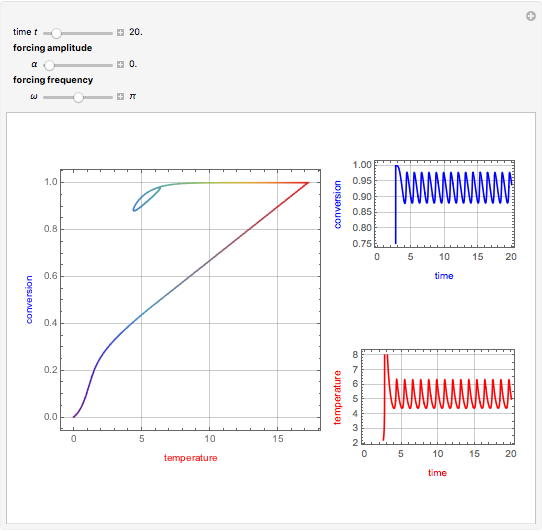
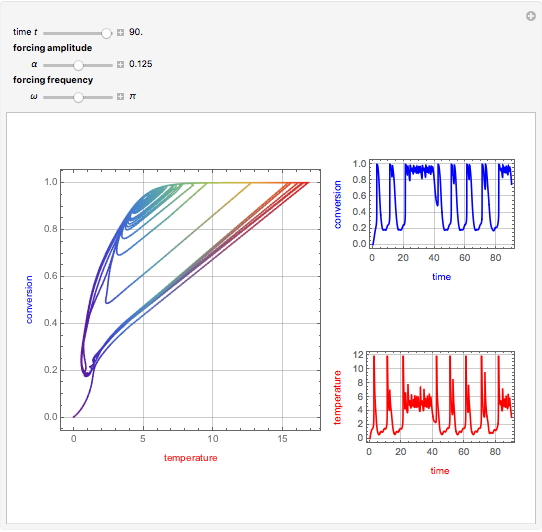
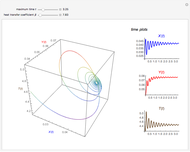
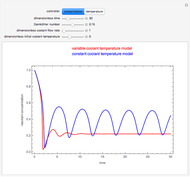
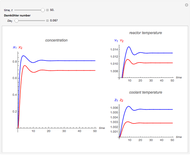
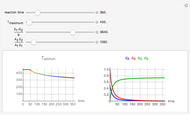
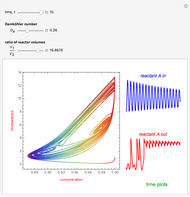
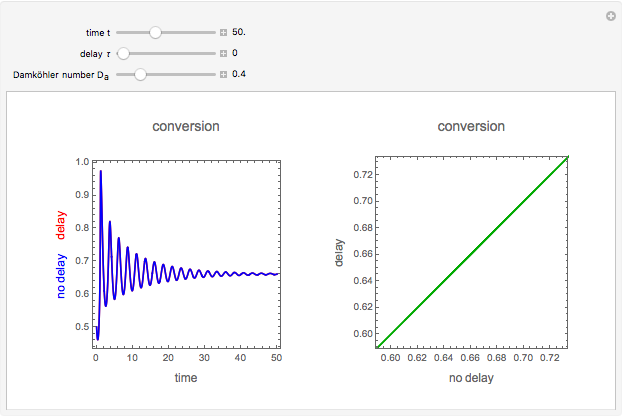
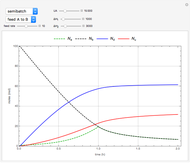
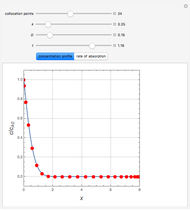
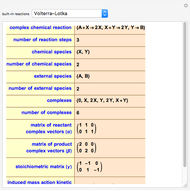
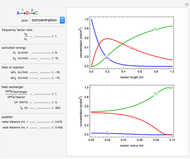
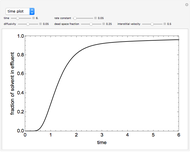
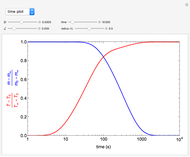
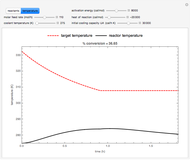
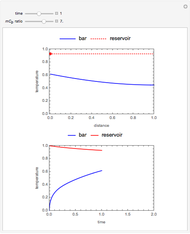
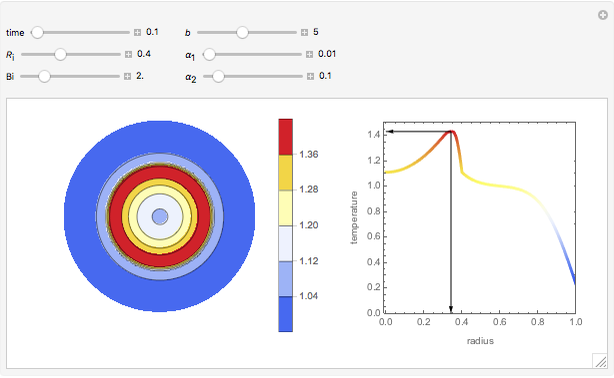
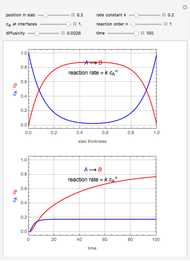
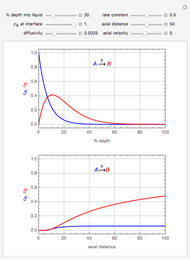
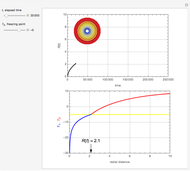
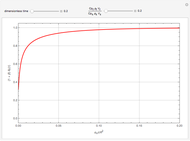
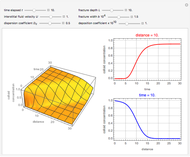
This Demonstration shows the effect of varying the coolant temperature of a continuous stirred-tank reactor in which an irreversible first-order exothermic chemical reaction  takes place.
takes place.
Contributed by: Clay Gruesbeck (January 2014)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] J. C. Mankin and J. L. Hudson, "Oscillatory and Chaotic Behavior of a Forced Exothermic Chemical Reaction," Chemical Engineering Science, 39(12), 1984 pp. 1807–1814.
Permanent Citation