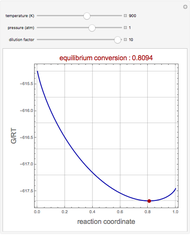
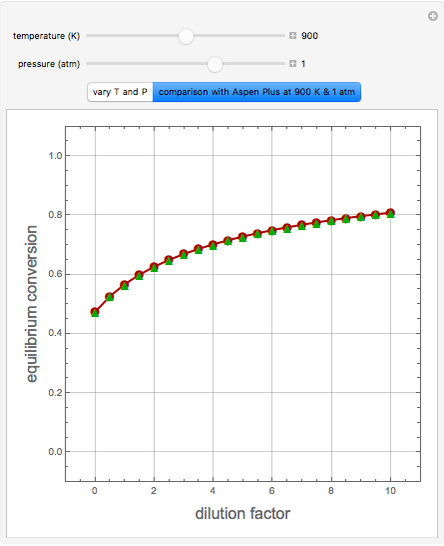
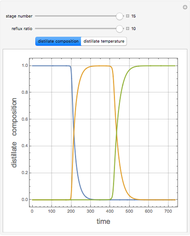
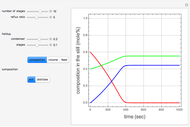
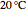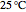Effect of Temperature on Solubility of Aniline-Methylcyclopentane-Hexane System

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
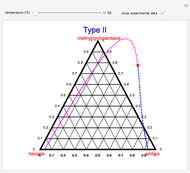
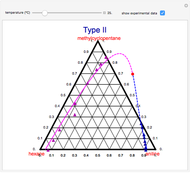
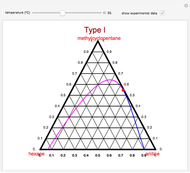
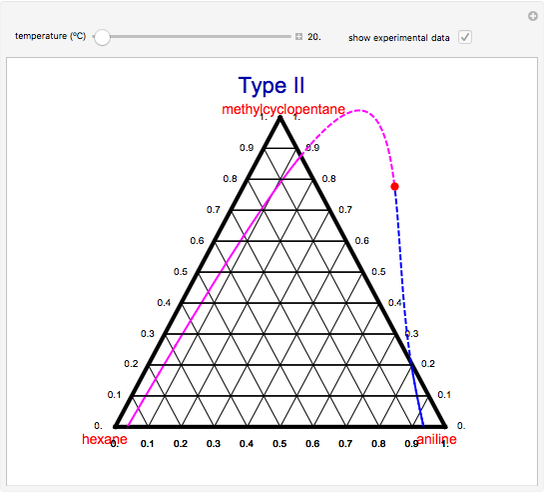
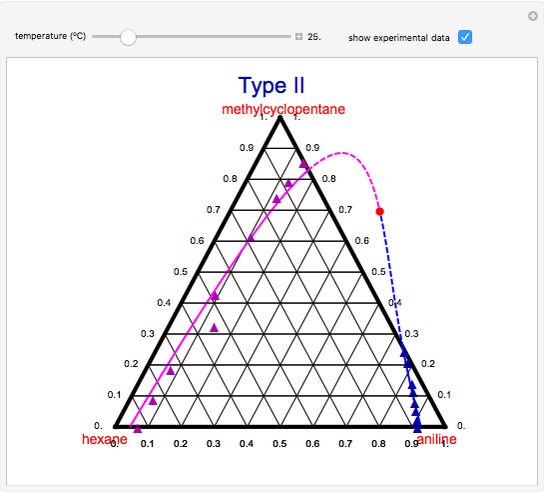
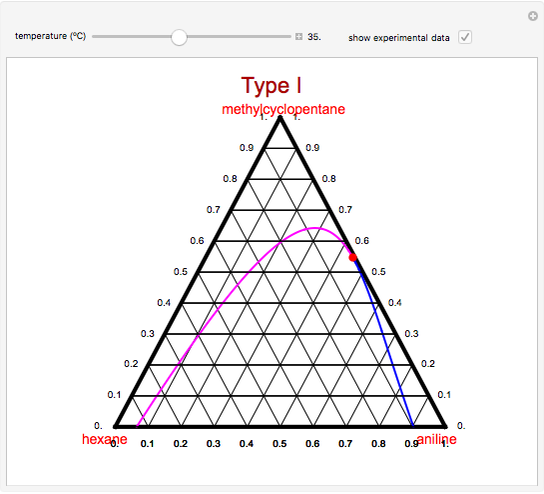
A ternary liquid system can be of Type I or Type II depending on the temperature 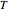 . This is especially true for the aniline-methylcyclopentane-
. This is especially true for the aniline-methylcyclopentane- -hexane system [1, 2]. This Demonstration uses arc-length continuation to compute the liquid-liquid equilibrium (LLE) diagram for this mixture. You can set the value of the temperature.
-hexane system [1, 2]. This Demonstration uses arc-length continuation to compute the liquid-liquid equilibrium (LLE) diagram for this mixture. You can set the value of the temperature.
Contributed by: Housam Binous, Farrukh Shehzad, Abdelmalek Hasseine, and Ahmed Bellagi (January 2016)
(King Fahd University of Petroleum & Minerals, KSA; University of Biskra, Algeria; University of Monastir, Tunisia)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References:
[1] B. de B. Darwent and C. A. Winkler, "The System  -Hexane–Methylcyclopentane–Aniline," The Journal of Physical Chemistry, 47(6), 1943 pp. 442–454. doi:10.1021/j150429a005.
-Hexane–Methylcyclopentane–Aniline," The Journal of Physical Chemistry, 47(6), 1943 pp. 442–454. doi:10.1021/j150429a005.
[2] J. D. Seader, E. J. Henley, and D. K. Roper, Separation Process Principles: Chemical and Biochemical Operations, 3rd ed., Hoboken, NJ: Wiley, 2011.
Permanent Citation