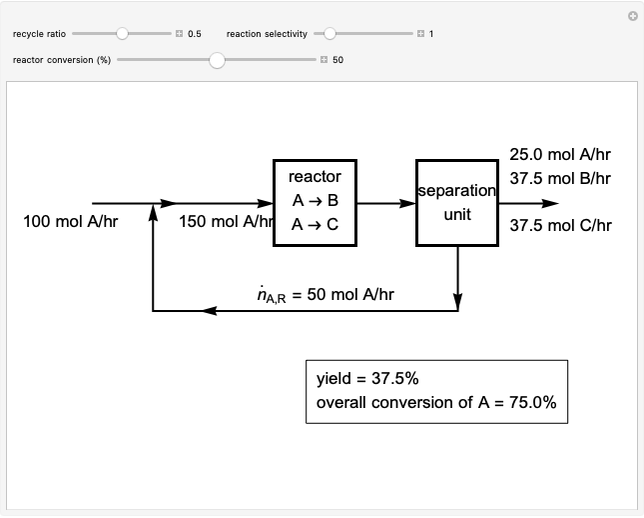
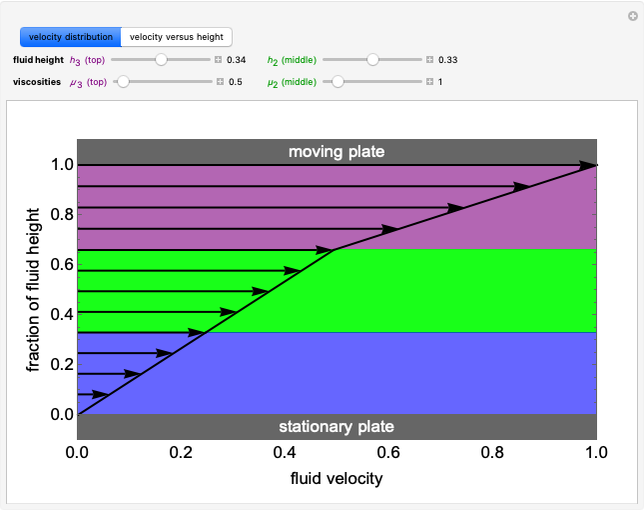
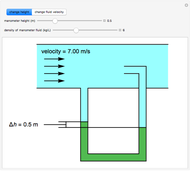
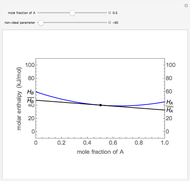
Evaporative Cooling of Water

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
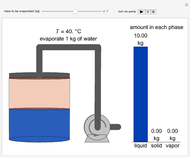
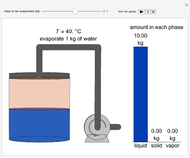
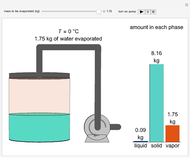
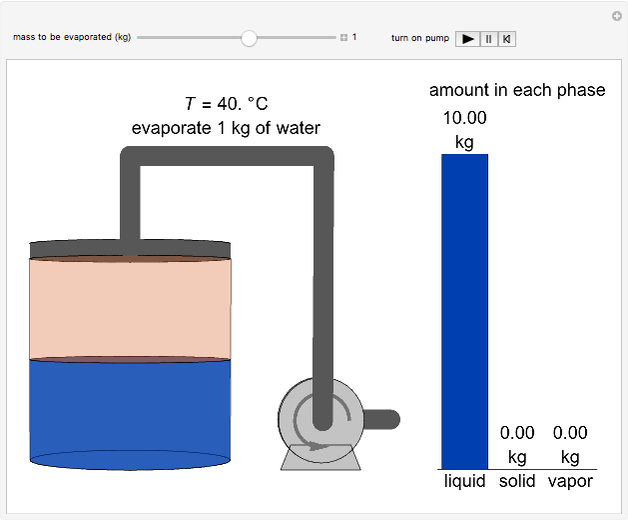
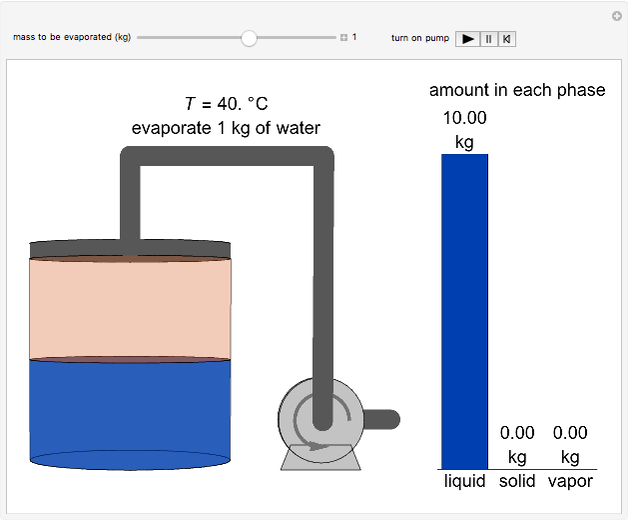
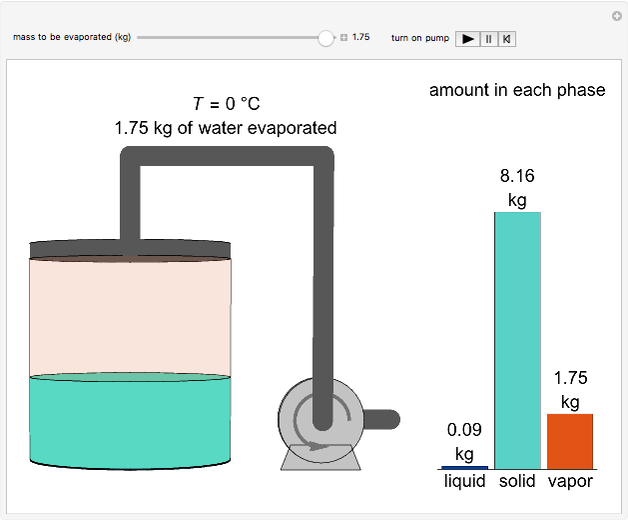
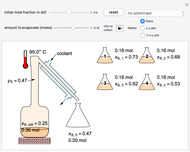
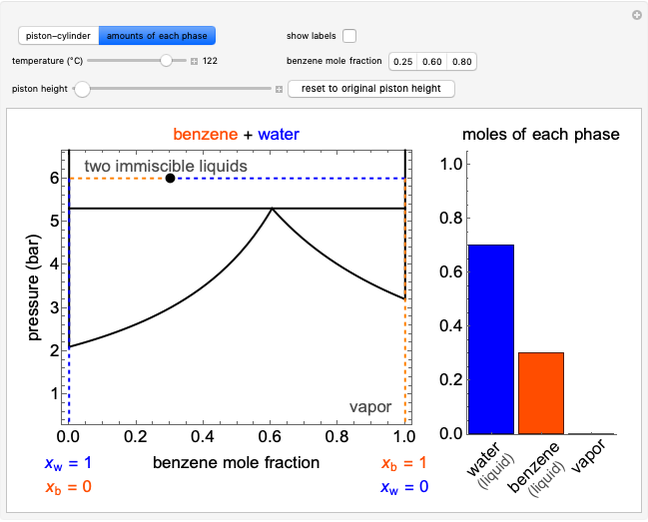
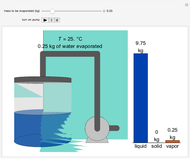
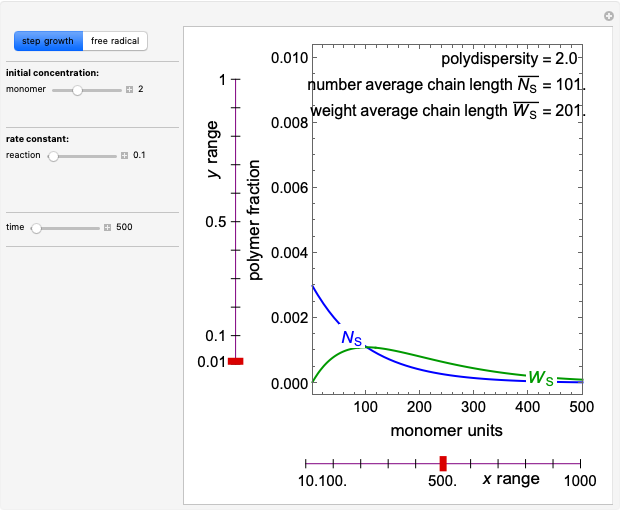
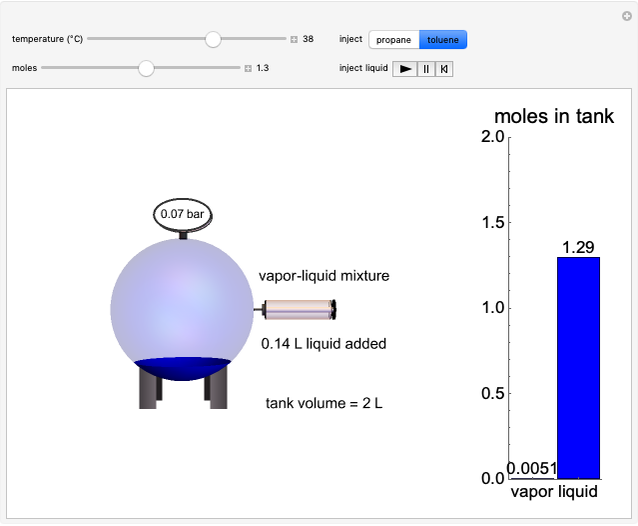
This Demonstration simulates the behavior when the vapor above a liquid is quickly removed from a tank by a pump. First, use the slider to select the mass of water to evaporate. Then, click the play button "turn on pump" to turn on a vacuum pump that removes water vapor from a well-insulated tank that initially contains 10 kg of liquid water at 40 °C. Water evaporates because the pump decreases the water partial pressure below its saturation pressure. The energy to evaporate the water is obtained by cooling the remaining liquid water, and then freezing some of the liquid when the temperature reaches 0 °C. The bar graph on the right shows the amounts of liquid and solid water in the tank and the amount of vapor that evaporated. In the tank, the remaining liquid is blue and the solid is light blue. The amounts of each phase are determined using unsteady-state mass and energy balances. To observe the behavior when a different amount evaporates, first click reset.
Contributed by: Rachael L. Baumann (June 2015)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Details
10 kg of liquid water are cooled from an initial temperature of 40 °C. An energy balance is used to determine the final temperature 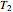 :
:
 ,
,
where 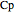 is the liquid heat capacity (kJ/[kg °C]),
is the liquid heat capacity (kJ/[kg °C]), 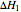 is the heat of vaporization at 20 °C (average of initial temperature and 0 °C) in kJ/kg and
is the heat of vaporization at 20 °C (average of initial temperature and 0 °C) in kJ/kg and 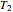 is in °C.
is in °C.
The amount of liquid 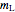 is 10 kg minus the amount of water vaporized
is 10 kg minus the amount of water vaporized 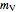 (both in kg):
(both in kg):
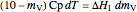 .
.
Integrating between the initial conditions (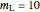 ,
, 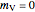 ) and the final conditions yields:
) and the final conditions yields:
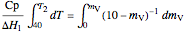 ,
,
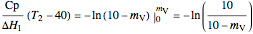 .
.
Thus the final temperature for a given amount of water vaporized is:
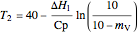 , where
, where 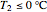 .
.
When 0.66 kg of water vaporizes, the liquid temperature is 0 °C and additional vaporization freezes some of the remaining liquid. An energy balance at 0 °C determines the amount of liquid that forms solid ice:
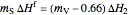 .
.
Here  is the mass of ice formed (kg),
is the mass of ice formed (kg), 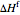 is the heat of fusion (kJ/kg) and
is the heat of fusion (kJ/kg) and  is the heat of vaporization at 0 °C (kJ/kg).
is the heat of vaporization at 0 °C (kJ/kg).
The screencast video at [1] explains how to use this Demonstration.
Reference
[1] Evaporative Cooling of Water [Video]. (Sep 3, 2019) http://www.learncheme.com/simulations/thermodynamics/thermo-1/evaporative-cooling-of-water.
Snapshots
Permanent Citation