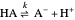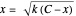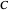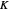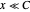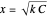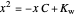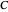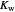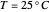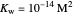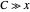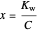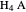Exact and Approximate Calculations of Acid-Base Equilibria

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
In this Demonstration, we show exact and approximate calculations for acid-base equilibria.
[more]
Contributed by: A. Ratti, D. Meliga, L. Lavagnino and S. Z. Lavagnino (June 2021)
Open content licensed under CC BY-NC-SA
Snapshots
Details
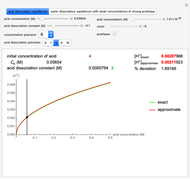
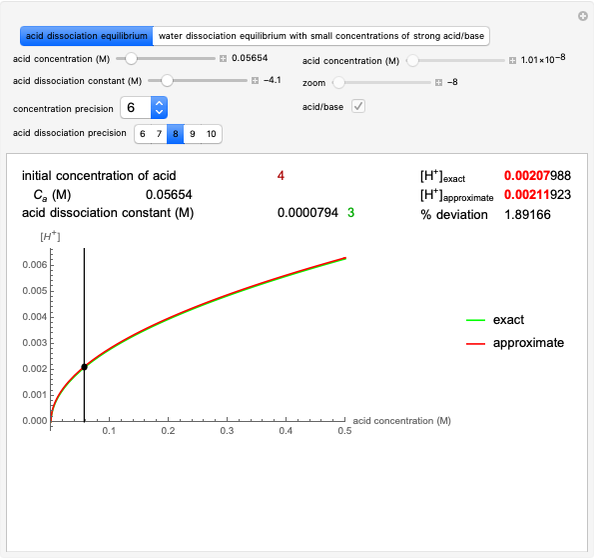
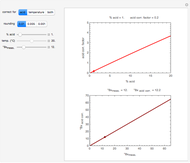
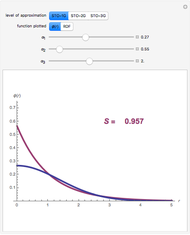
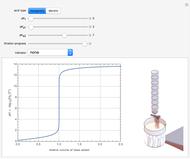
Snapshot 1: Exact and approximate equations give the same result since the initial concentration is high and the extent of dissociation is very low (weak acid).
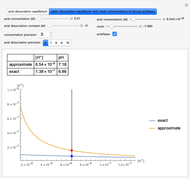
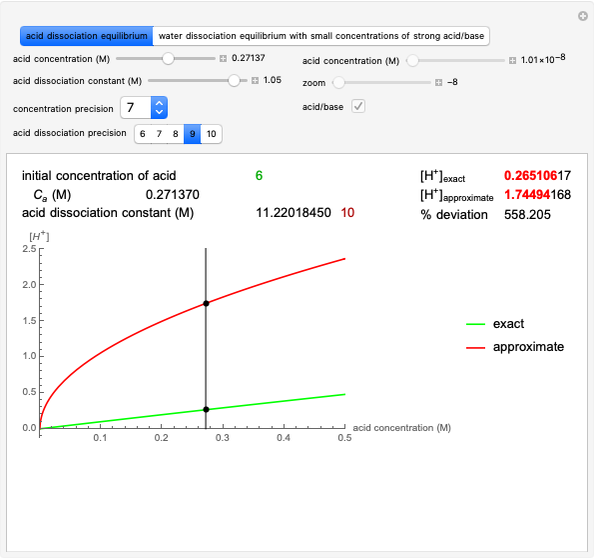
Snapshot 2: Exact and approximate equations are very different since we have a very high acid dissociation constant. In this case, the acid is completely dissociated (strong acid).
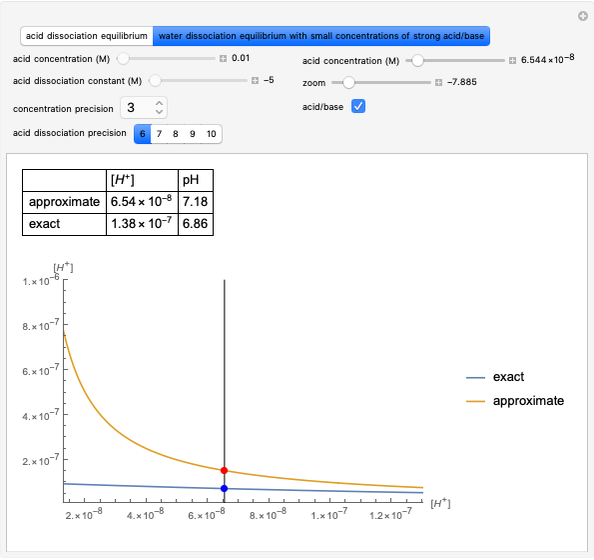
Snapshot 3: Incorrect use of the approximate equation gives the pH of a base although the solution is a slightly acidic.
Reference
[1] D. C. Harris, Quantitative Chemical Analysis, 8th ed., New York: W. H. Freeman and Company, 2010.
Permanent Citation