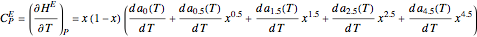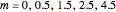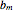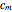Excess Enthalpy and Heat Capacity for an Ethanol-Water Mixture

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
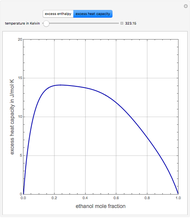
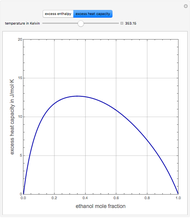
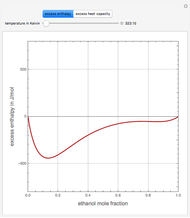
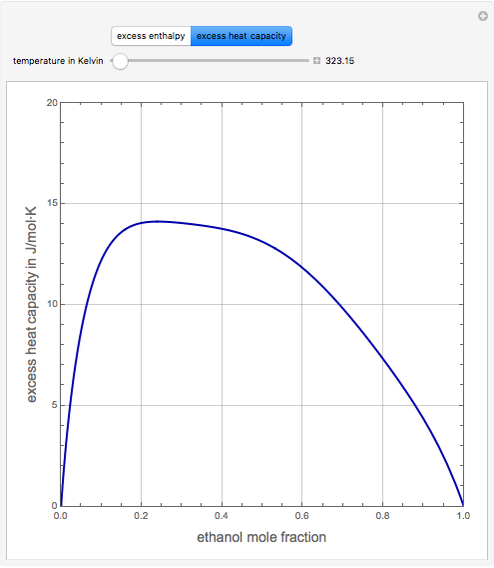
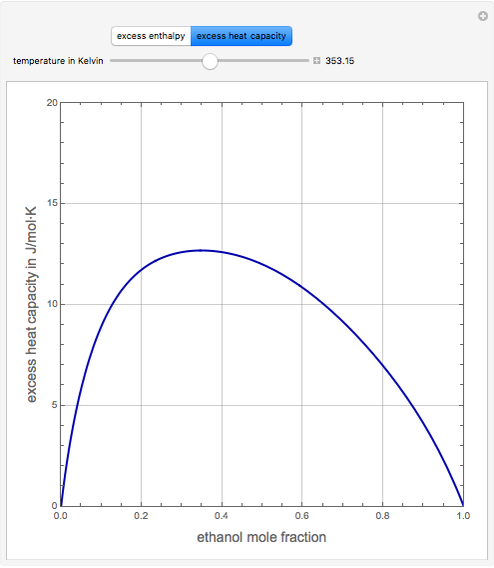
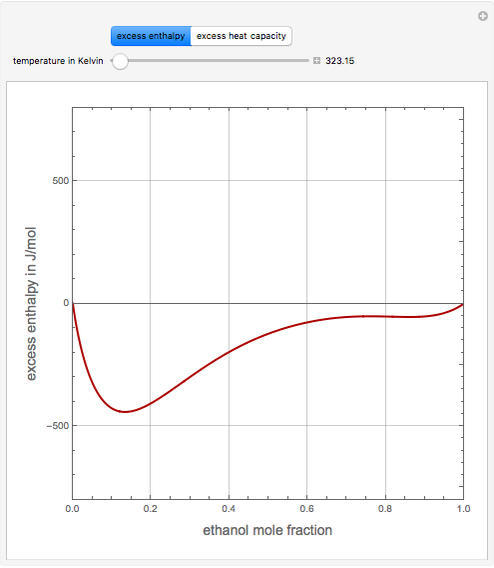
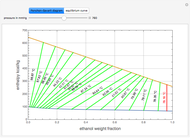
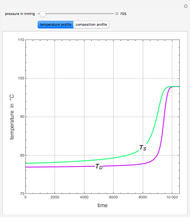
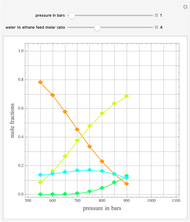
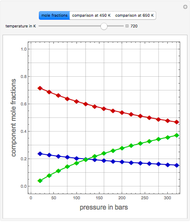
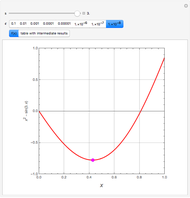
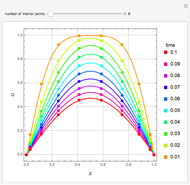
Consider a binary mixture of ethanol and water. Expressions for the excess enthalpy (in J/mol) and heat capacity (in J/mol·K) have been determined for this binary liquid system for the temperature range 298.15 K to 383.15 K by fitting experimental data to an empirical equation [1].
[more]
Contributed by: Housam Binous and Brian G. Higgins (January 2012)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] J. A. Larkin, "Thermodynamic Properties of Aqueous Non-Electrolyte Mixtures I. Excess Enthalpy for Water + Ethanol at 298.15 to 383.15 K," Journal of Chemical Thermodynamics, 7(2), 1975 pp. 137–148. doi:10.1016/0021-9614(75)90261-X.
Permanent Citation
"Excess Enthalpy and Heat Capacity for an Ethanol-Water Mixture"
http://demonstrations.wolfram.com/ExcessEnthalpyAndHeatCapacityForAnEthanolWaterMixture/
Wolfram Demonstrations Project
Published: January 5 2012