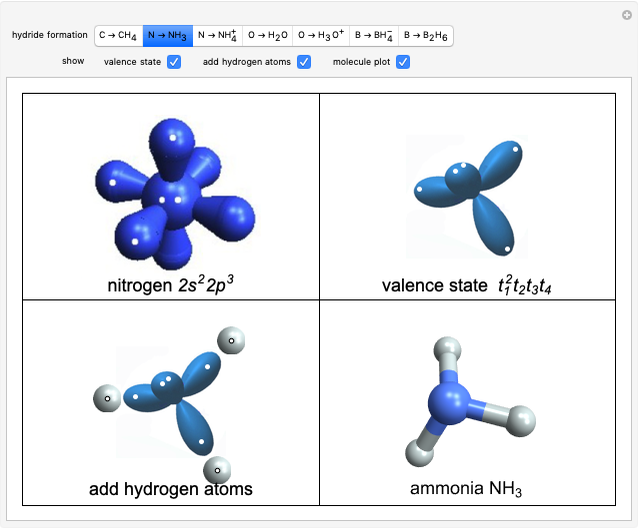
Filling the Gaps in Mendeleev's Periodic Table

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
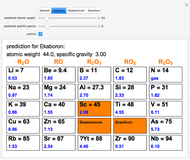
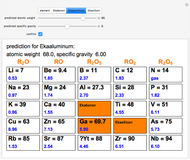
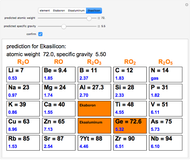
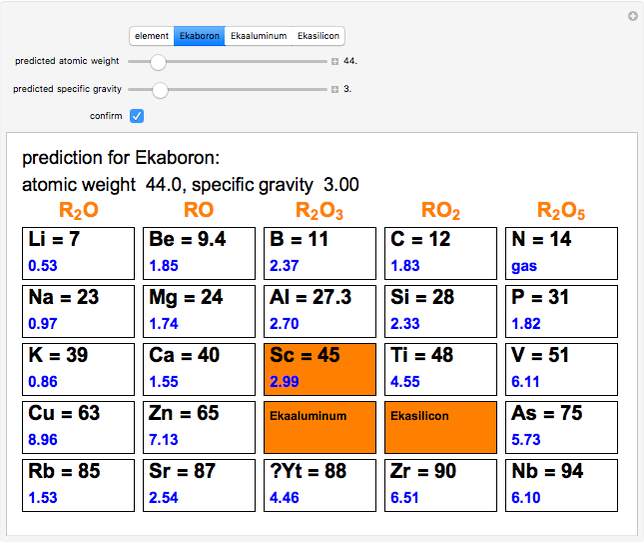
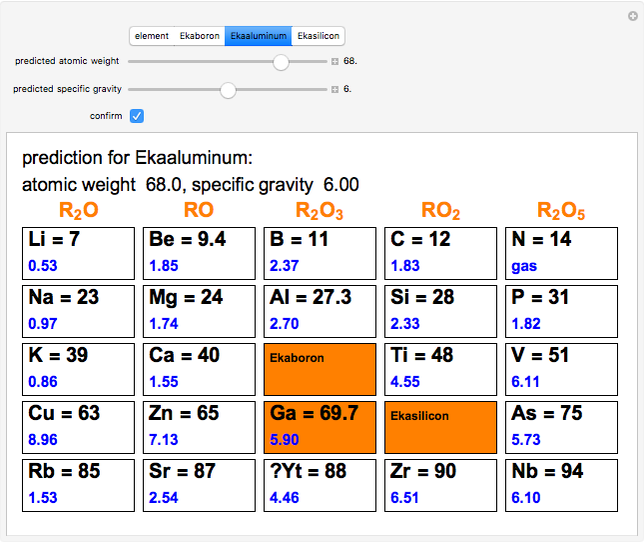
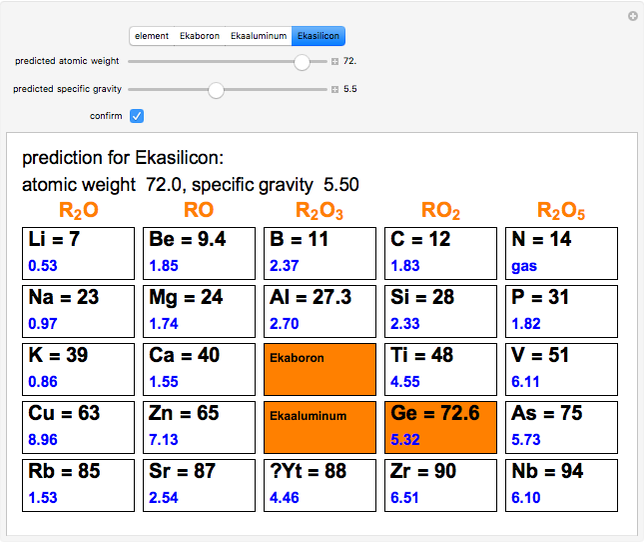
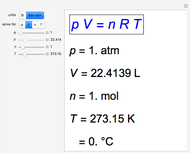
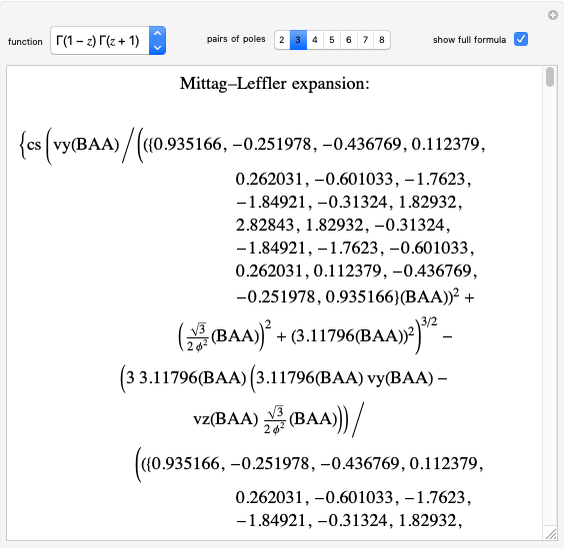
The graphic shows a fragment of the periodic table of the elements published by Dmitri Ivanovich Mendeleev in 1871. The elements are ordered by increasing atomic weight, such that elements with chemical similarities occur in vertical groups. The headings give the formulas for the most stable oxide for elements in each group. Also shown in blue are the specific gravities of the elements. Mendeleev boldly proposed that there must be missing elements in the three orange boxes, to which he gave the provisional names Ekaboron, Ekaaluminum and Ekasilicon. Try to estimate the atomic weights and specific gravities for these elements. Scandium, Gallium and Germanium were discovered in 1879, 1875 and 1886, respectively, with properties very close to those predicted by Mendeleev. It is now understood, of course, that it is more fundamental to order the elements by atomic number, rather than atomic weight.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshots 1–3: Mendeleev's predictions for atomic weight and specific gravity of Ekaboron, Ekaaluminum and Ekasilicon, compared to observed properties of scandium (Sc), gallium (Ga) and germanium (Ge)
Reference: S. M. Blinder, Introduction to Quantum Mechanics, Amsterdam: Elsevier, 2004 p. 124.
Permanent Citation
"Filling the Gaps in Mendeleev's Periodic Table"
http://demonstrations.wolfram.com/FillingTheGapsInMendeleevsPeriodicTable/
Wolfram Demonstrations Project
Published: March 7 2011