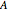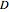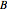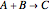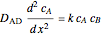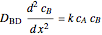Gas Absorption with Chemical Reactions
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
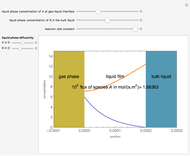
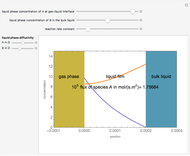
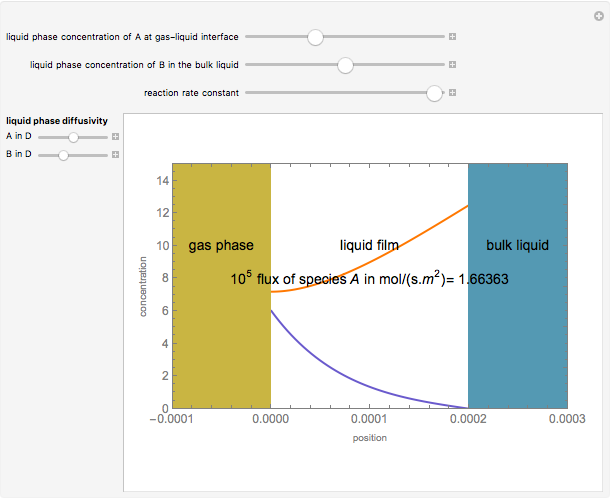
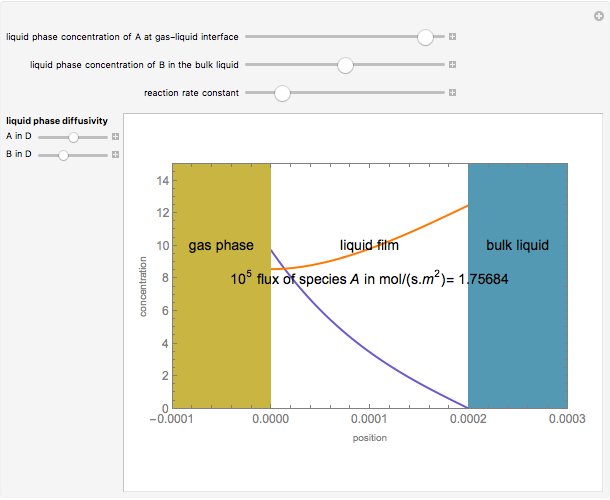
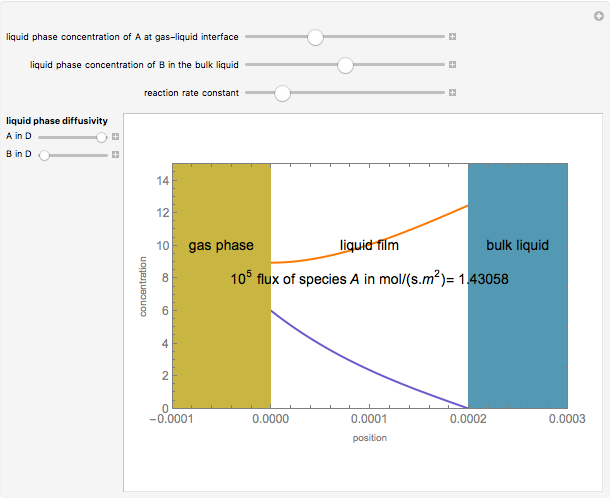
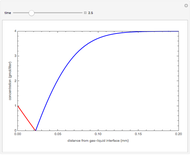
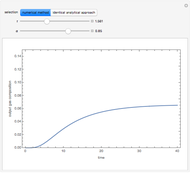
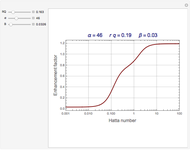
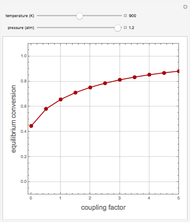
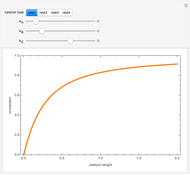
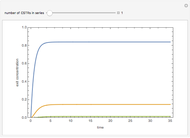
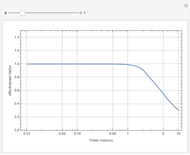
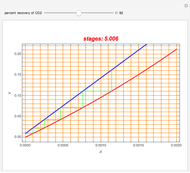
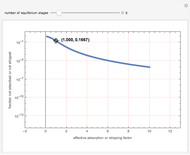
Gas absorption is often enhanced by chemical reaction. For instance, acid gases (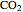 and
and 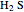 ) are usually eliminated from natural gas with absorption using ethanolamine (
) are usually eliminated from natural gas with absorption using ethanolamine (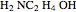 ) as a basic solvent.
) as a basic solvent.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
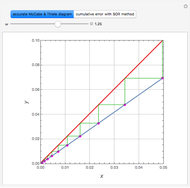
Reference: M. B. Cutlip and M. Shacham, Problem Solving in Chemical Engineering with Numerical Methods, Upper Saddle River, N.J.: Prentice Hall, 1999 (Example 7.11, Second-Order Reaction with Diffusion in Liquid Film).
Permanent Citation