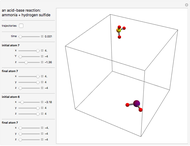
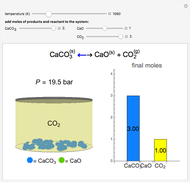
Gas-Phase Chemical Equilibrium at Constant Pressure or Constant Volume

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
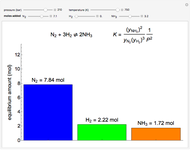
This Demonstration calculates the number of moles at equilibrium for a gas-phase reaction 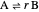 at constant temperature; the equilibrium constant for the reaction is
at constant temperature; the equilibrium constant for the reaction is  . Components
. Components  and
and 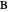 are ideal gases, and you can set the value of
are ideal gases, and you can set the value of  (1/2, 1, 3/2, or 2) with buttons. Initially the container is filled with 5 mol of reactant
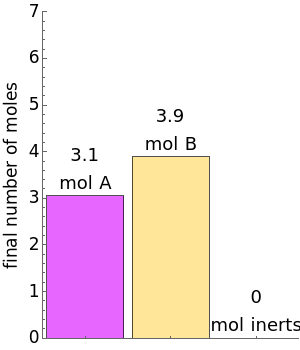
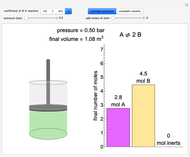
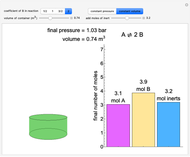
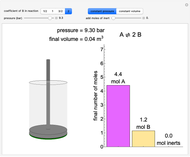
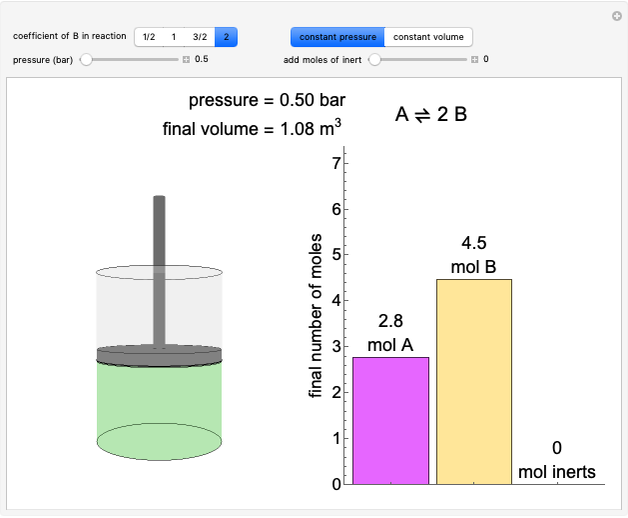
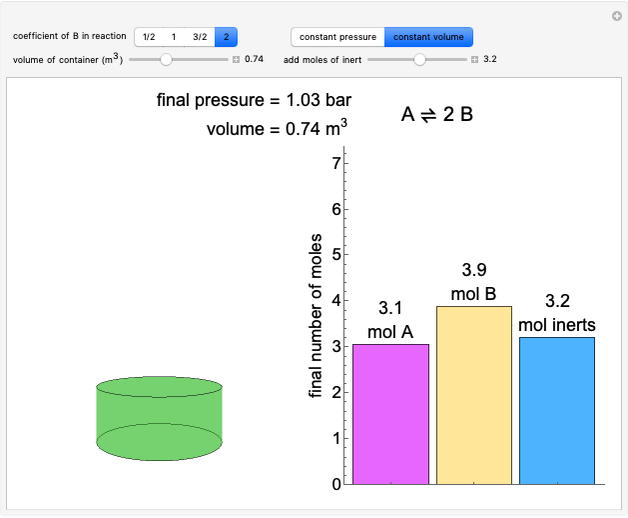
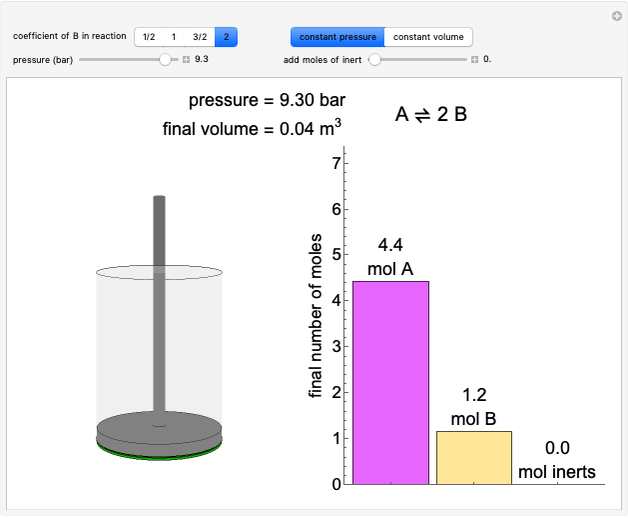
(1/2, 1, 3/2, or 2) with buttons. Initially the container is filled with 5 mol of reactant 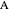 , and equilibrium is obtained at either constant pressure (set pressure with a slider) or constant volume (set volume with a slider). The bar graph displays the number of moles at equilibrium, including the moles of inert gas (select the moles of inert with a slider). The height of the piston or container is proportional to the final volume.
, and equilibrium is obtained at either constant pressure (set pressure with a slider) or constant volume (set volume with a slider). The bar graph displays the number of moles at equilibrium, including the moles of inert gas (select the moles of inert with a slider). The height of the piston or container is proportional to the final volume.
Contributed by: Garrison J. Vigil and Rachael L. Baumann (July 2015)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
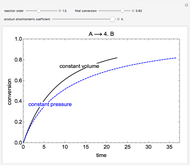
The number of moles of each species in this reaction at equilibrium (values shown on the chart) determines the extent of reaction  :
:
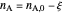 ,
,
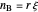 ,
,
where  and
and 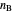 are the moles of reactant and product at equilibrium (mol),
are the moles of reactant and product at equilibrium (mol),  is the moles of reactant present initially, and
is the moles of reactant present initially, and  is the ratio of moles of product to moles of reactant.
is the ratio of moles of product to moles of reactant.
The equilibrium constant  is:
is:
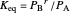 ,
,
where 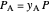 is the partial pressure of the reactant,
is the partial pressure of the reactant,  is the partial pressure of the product, and
is the partial pressure of the product, and  is the total pressure (bar).
is the total pressure (bar).
The mole fraction of each species at equilibrium is:
 ,
,
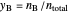 ,
,
where the total number of moles is  , with
, with 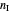 the number of moles of any inert component in the mixture.
the number of moles of any inert component in the mixture.
The extent of reaction is found by setting 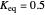 and solving for
and solving for  .
.
The screencast video at [1] shows how to use this Demonstration.
Reference
[1] Gas-Phase Chemical Equilibrium at Constant Pressure or Constant Volume [Video]. (Dec 16, 2020) www.learncheme.com/simulations/thermodynamics/thermo-2/gas-phase-chemical-equilibrium-at-constant-p-or-constant-v.
Permanent Citation