Gay-Lussac's or Amontons's Law for an Ideal Gas
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
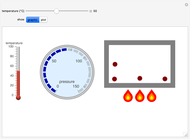
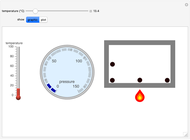
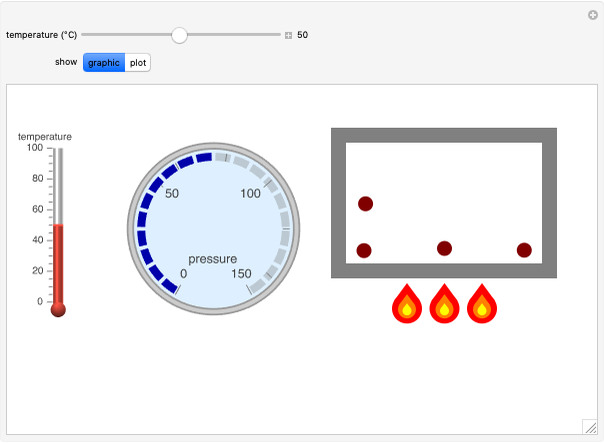
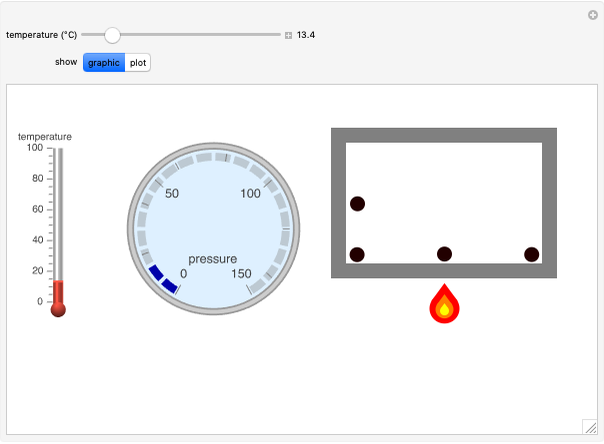
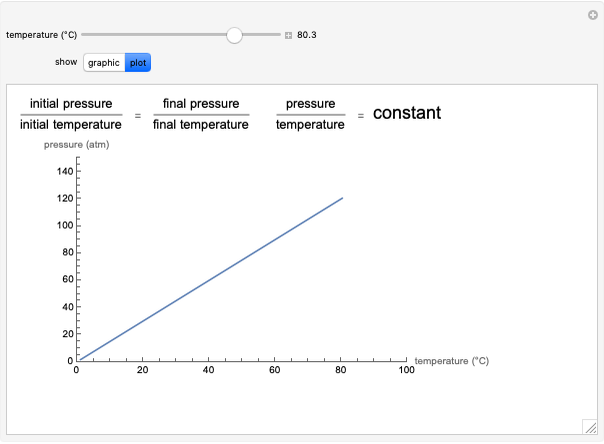
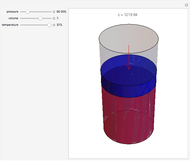
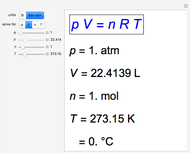
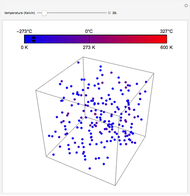
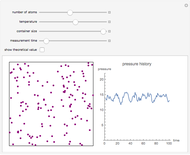
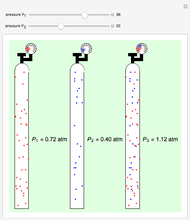
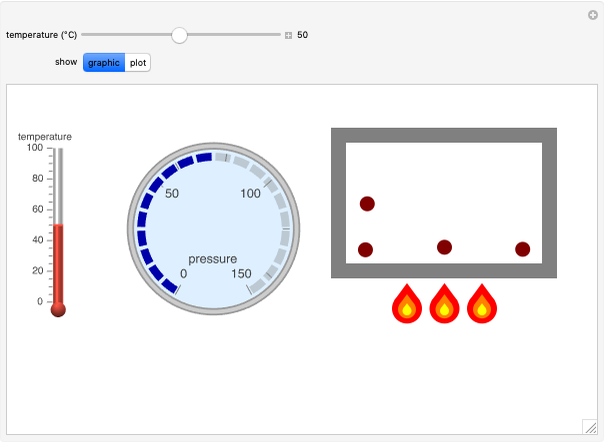
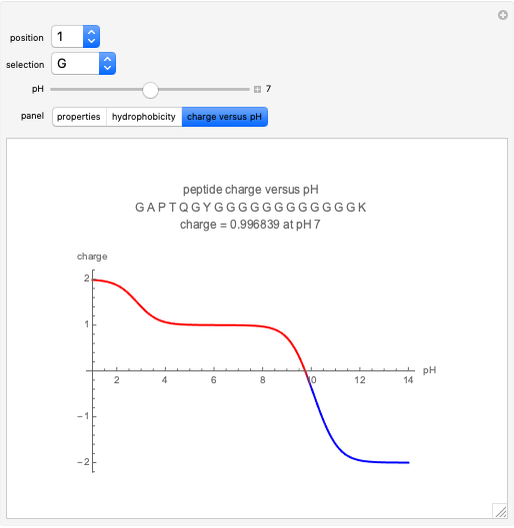
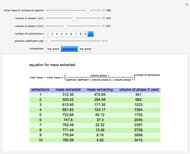
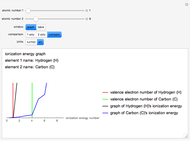
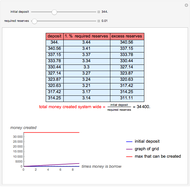
Gay-Lussac's law (or Amontons's law) states that the temperature and pressure of a gas are linearly related. As the temperature increases, so does the pressure, as shown in the plot. The graphic gives an idealized picture of the molecular motion associated with changes of temperature and pressure.
Contributed by: Alla Ahmad (January 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Permanent Citation