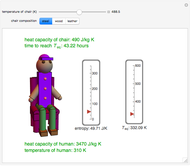
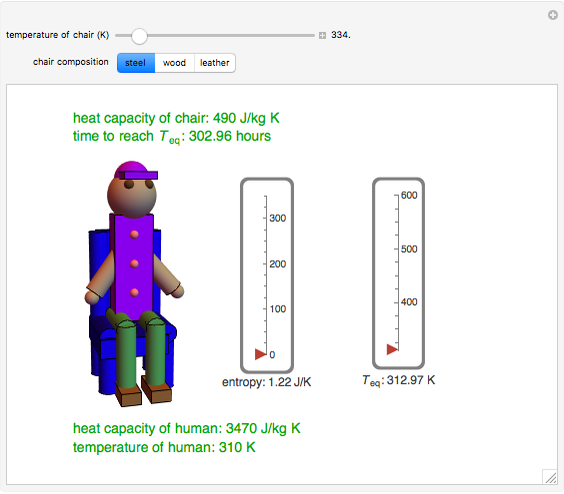
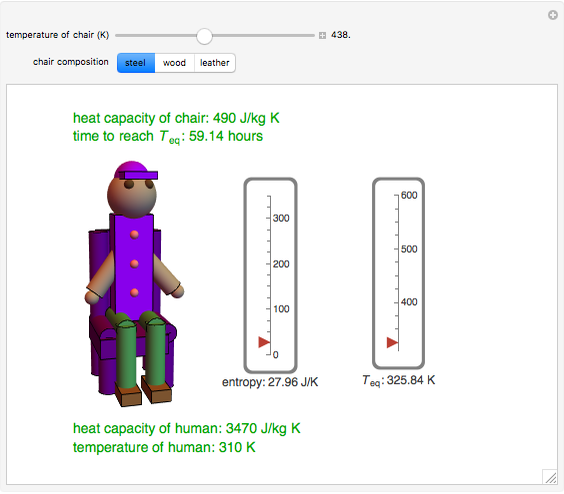
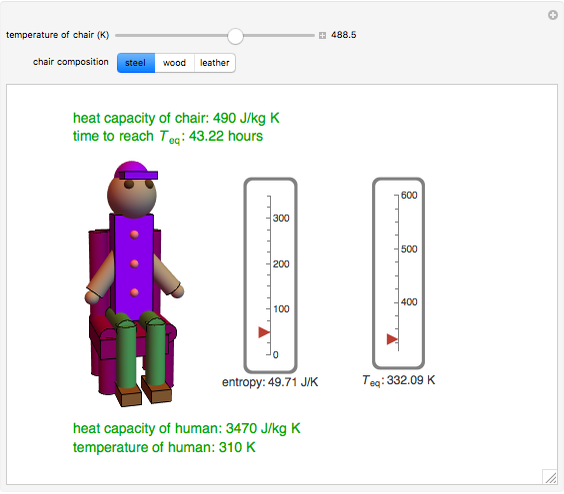
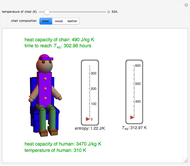
Heat Flow between a Human and a Chair
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
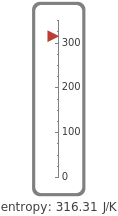
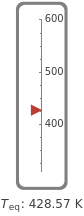
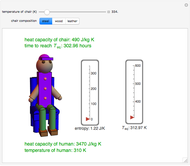
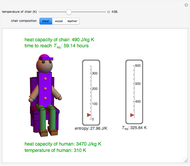
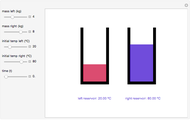
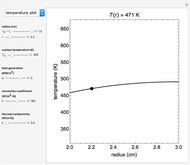
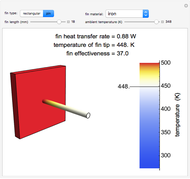
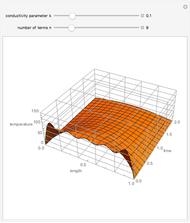
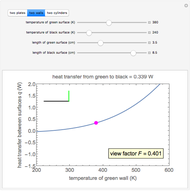
This Demonstration shows the equilibration between two objects at different temperatures in thermal contact with one another. In this case, the objects are a human and a chair of equal mass.
[more]
Contributed by: Abhi Krishnaraj (December 2016)
Additional contributions by: Eitan Geva (University of Michigan)
Open content licensed under CC BY-NC-SA
Details
Submission from the Compute-to-Learn course at the University of Michigan.
Snapshots
Permanent Citation