Heating Water and Air in a Sealed Container

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
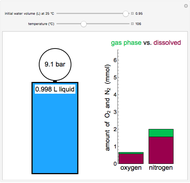
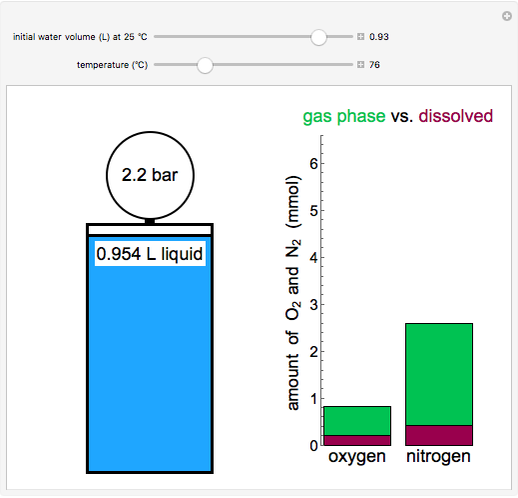
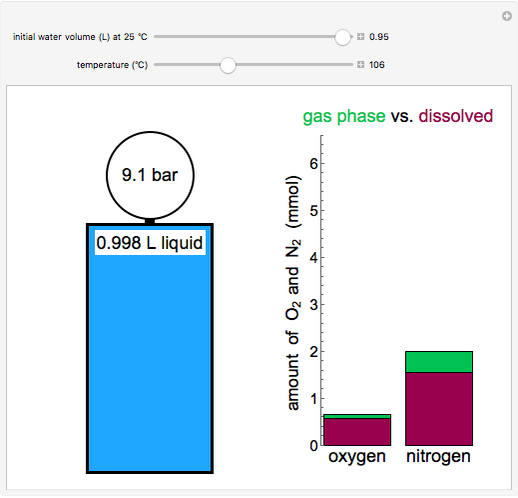
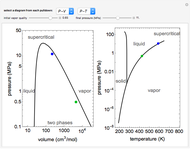
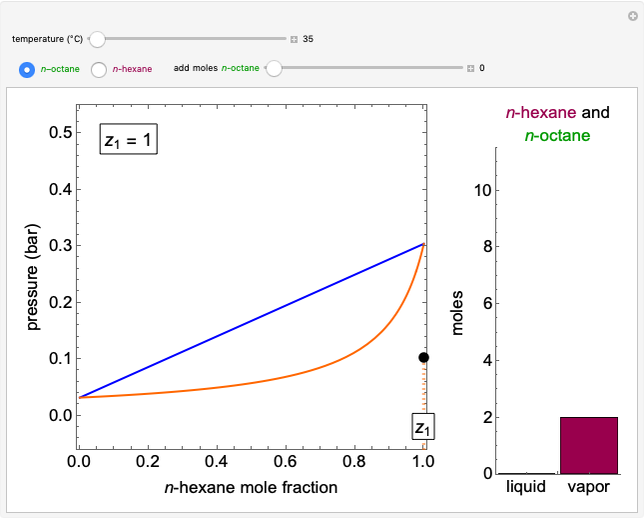
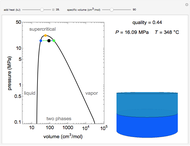
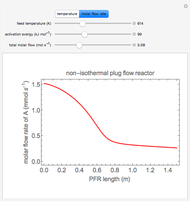
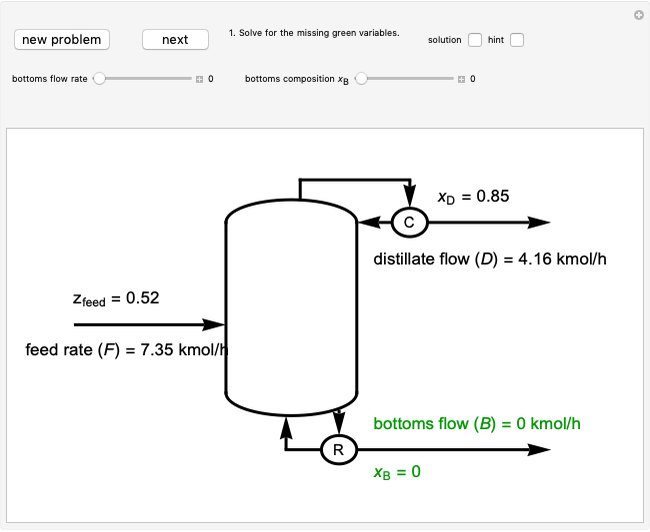
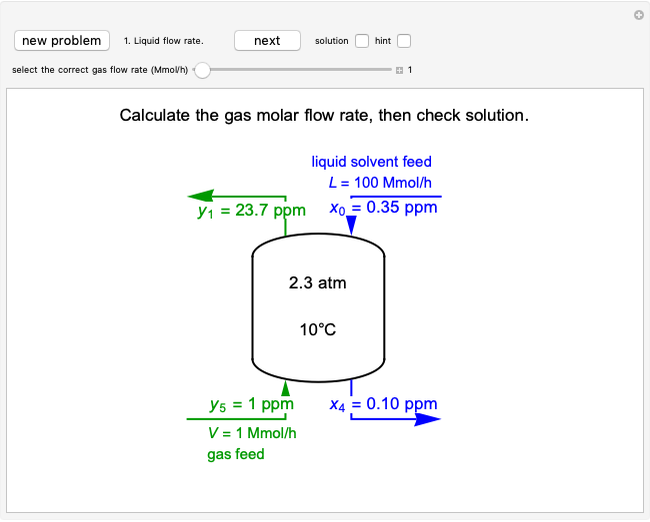
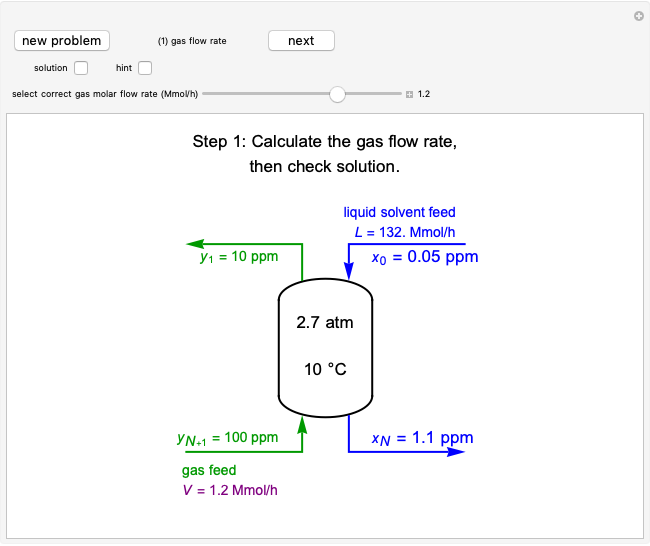
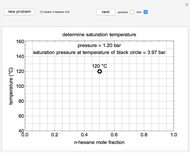
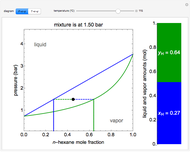
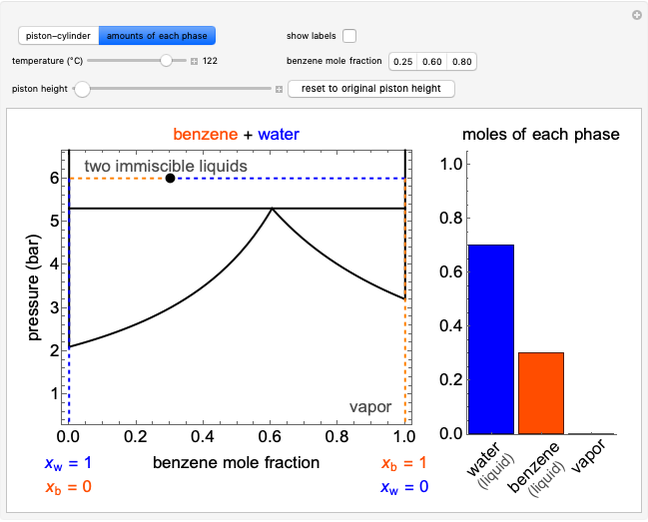
This Demonstration models the behavior of a sealed, 1-L autoclave that initially contains mostly water plus a small volume of air, all at 25 °C. Change the initial volume of water with a slider and the temperature resets to 25 °C. As the temperature increases by moving the slider, liquid water expands and its saturation pressure increases. At the same time, the gas-phase volume decreases, so gas-phase 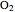 and
and 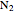 partial pressures increase (ideal gas law). The amounts of
partial pressures increase (ideal gas law). The amounts of 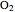 and
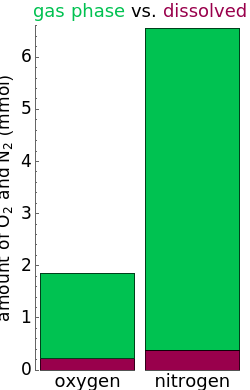
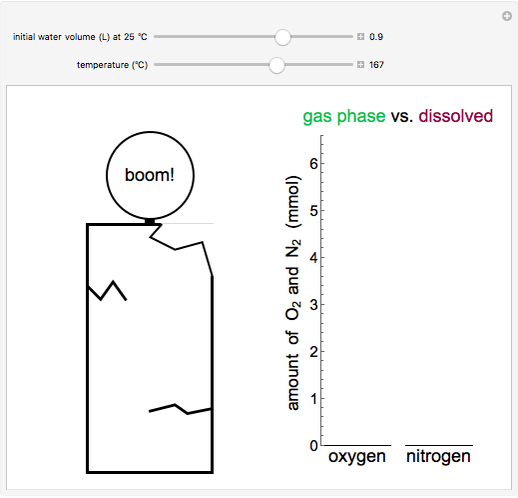
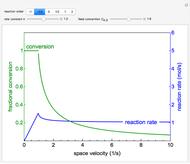
and 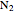 dissolved in the water increase with pressure, but decrease with temperature. The amounts dissolved are shown in the bar chart (green = gas phase, purple = dissolved). Even at moderate temperatures, the pressure inside the sealed container can be quite high.
dissolved in the water increase with pressure, but decrease with temperature. The amounts dissolved are shown in the bar chart (green = gas phase, purple = dissolved). Even at moderate temperatures, the pressure inside the sealed container can be quite high.
Contributed by: Derek M. Machalek and Rachael L. Baumann (August 2015)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The final liquid volume 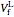 of water is given by:
of water is given by:
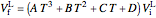 ,
,
where 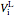 is the initial liquid volume (L) at 25 °C,
is the initial liquid volume (L) at 25 °C, 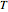 is temperature (°C), and
is temperature (°C), and 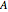 ,
, 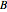 ,
, 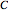 and
and 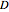 are constants.
are constants.
The total pressure 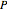 in the container is equal to the saturation pressure of water
in the container is equal to the saturation pressure of water 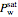 plus the partial pressures of oxygen
plus the partial pressures of oxygen 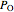 and nitrogen
and nitrogen 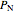 :
:
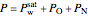 .
.
The saturation pressure of water is calculated using the Antoine equation:
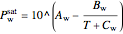 ,
,
where 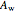 ,
, 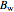 and
and 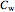 are Antoine constants.
are Antoine constants.
The partial pressures of oxygen and nitrogen are calculated using the ideal gas law:
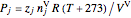 ,
,
where 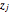 is the fraction of oxygen or nitrogen in air where
is the fraction of oxygen or nitrogen in air where 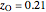 and
and 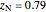 ,
, 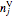 is the moles of
is the moles of 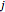 in the gas phase,
in the gas phase, 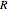 is the ideal gas constant ([L bar]/[mol K]) and
is the ideal gas constant ([L bar]/[mol K]) and 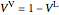 is the vapor volume (L).
is the vapor volume (L).
The total moles of oxygen 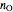 and nitrogen
and nitrogen 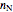 in the container are calculated at 25 °C and 1 bar pressure, the moles in the gas phase are calculated using the ideal gas law, and the moles dissolved in water are calculated using Henry's law:
in the container are calculated at 25 °C and 1 bar pressure, the moles in the gas phase are calculated using the ideal gas law, and the moles dissolved in water are calculated using Henry's law:
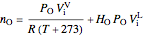 ,
,
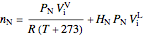 ,
,
where 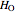 and
and 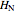 are Henry's law constants (mol/[L bar]):
are Henry's law constants (mol/[L bar]):
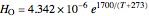 ,
,
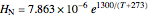 .
.
For all 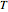 and
and 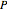 , the moles in the gas phase and dissolved must equal the total moles at 25 °C and 1 bar:
, the moles in the gas phase and dissolved must equal the total moles at 25 °C and 1 bar:
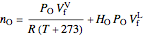 ,
,
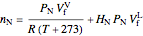 .
.
The screencast video at [1] shows how to use this Demonstration.
Reference
[1] Heating Water and Air in a Sealed Container [Video]. (Aug 31, 2016) www.colorado.edu/learncheme/thermodynamics/HeatingWaterAirSealedContainer.html.
Permanent Citation