Hydrogen Atom Radial Functions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
The eigenfunctions in spherical coordinates for the hydrogen atom are 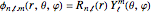 , where
, where 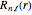 and
and 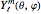 are the solutions to the radial and angular parts of the Schrödinger equation, respectively, and
are the solutions to the radial and angular parts of the Schrödinger equation, respectively, and  ,
,  , and
, and 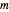 are the principal, orbital, and magnetic quantum numbers with allowed values
are the principal, orbital, and magnetic quantum numbers with allowed values 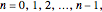
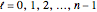 , and
, and 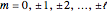 . The
. The 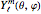 are the spherical harmonics and the radial functions are
are the spherical harmonics and the radial functions are 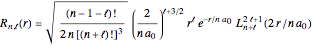 , where
, where 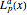 is the
is the 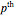 -order associated Laguerre polynomial and
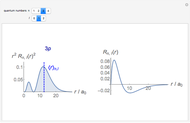
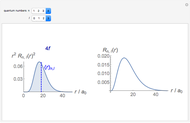
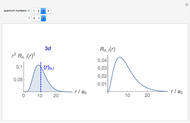
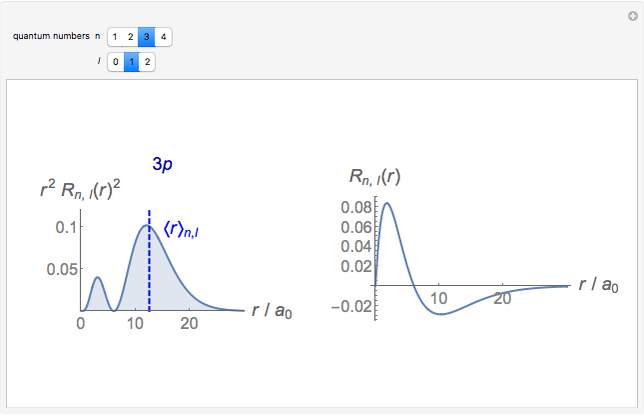
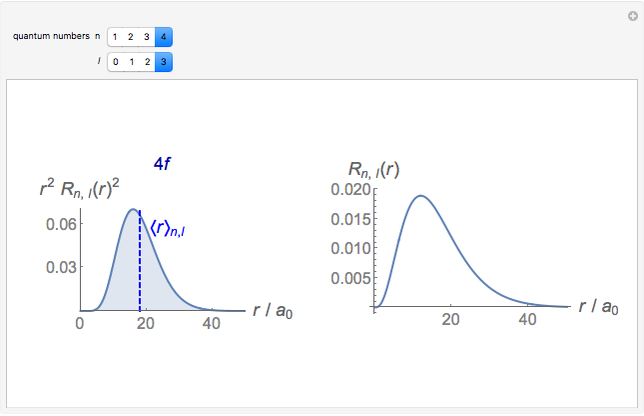
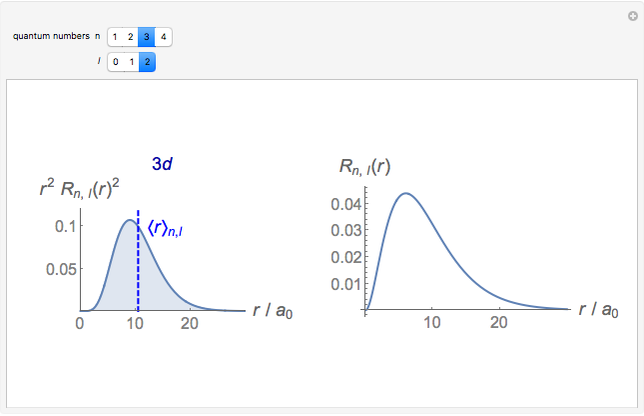
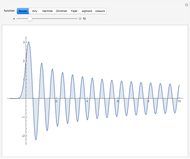
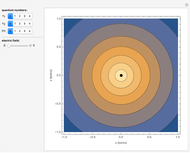
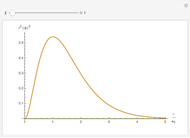
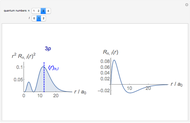
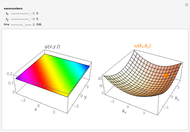
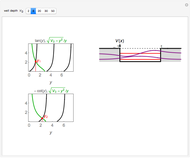
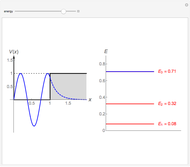
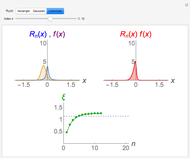
-order associated Laguerre polynomial and 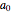 is the Bohr radius. The left graphic shows the radial probability density
is the Bohr radius. The left graphic shows the radial probability density 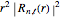 and the expectation value
and the expectation value 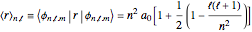 , and the right graphic shows the radial function.
, and the right graphic shows the radial function.
Contributed by: Porscha McRobbie and Eitan Geva (January 2010)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Hydrogen Atom Radial Functions"
http://demonstrations.wolfram.com/HydrogenAtomRadialFunctions/
Wolfram Demonstrations Project
Published: January 8 2010