Ionization Energies of Elements up to Z=36

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
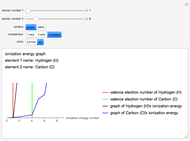
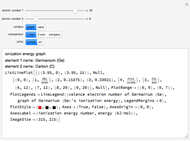
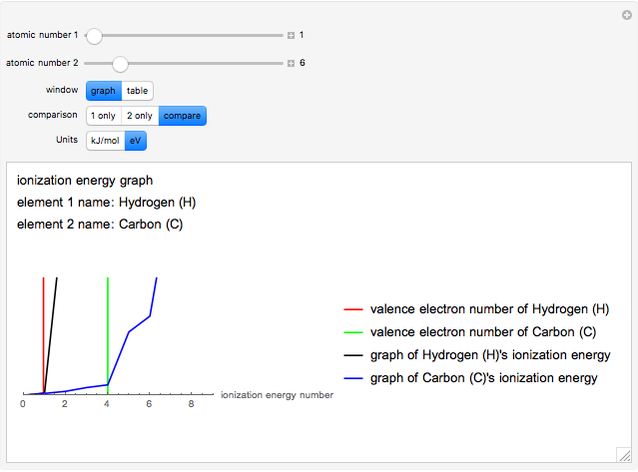
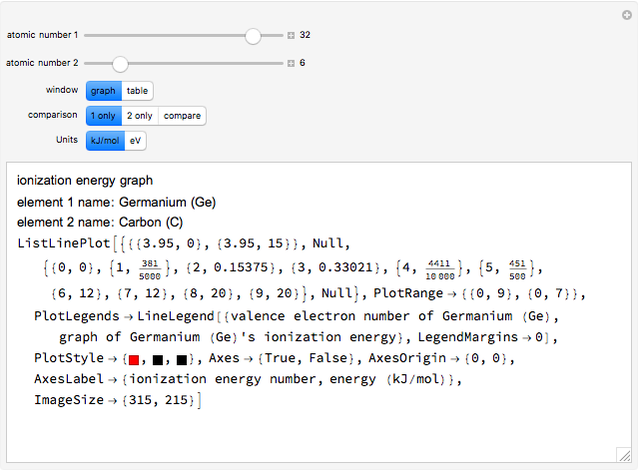
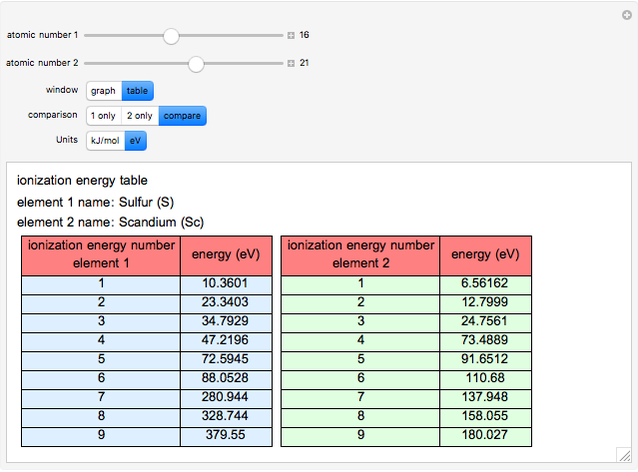
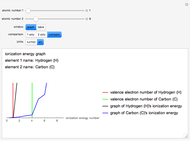
The ionization energy of an element is the energy required to remove the outermost electron of a gaseous atom to infinity. The  ionization energy is the energy to remove the outermost electron remaining after
ionization energy is the energy to remove the outermost electron remaining after  electrons have been removed. Ionization energies depend predominantly on the atomic number
electrons have been removed. Ionization energies depend predominantly on the atomic number  of an element (the charge of the nucleus) and the electron's average distance from the nucleus, with shorter distance giving larger ionization energy. The peaking seen in the graphs usually occurs after the vertical line corresponding to the number of electrons in the valence (outermost) shell, before electrons are removed from the next innermost shell, which have smaller average distances from the nucleus than those in the valence shell. Ionization energy is correlated with many other properties of an element, including the existence of stable cations, the reactivity of the element, and its possible oxidation states.
of an element (the charge of the nucleus) and the electron's average distance from the nucleus, with shorter distance giving larger ionization energy. The peaking seen in the graphs usually occurs after the vertical line corresponding to the number of electrons in the valence (outermost) shell, before electrons are removed from the next innermost shell, which have smaller average distances from the nucleus than those in the valence shell. Ionization energy is correlated with many other properties of an element, including the existence of stable cations, the reactivity of the element, and its possible oxidation states.
Contributed by: Alla Ahmad (June 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
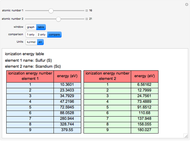
Ionization energies were taken from [1]. Only the first periodicity is listed; there are also second to ninth.
References
[1] M. Winter. "Ionization energy: 1st: periodicity." WebElements. (2015) http://www.webelements.com/periodicity/ionisation_energy_ 1.
Permanent Citation
"Ionization Energies of Elements up to Z=36"
http://demonstrations.wolfram.com/IonizationEnergiesOfElementsUpToZ36/
Wolfram Demonstrations Project
Published: June 23 2015