Isothermal Plug Flow Reactor

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
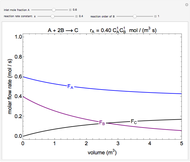
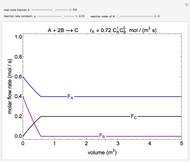
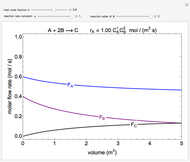
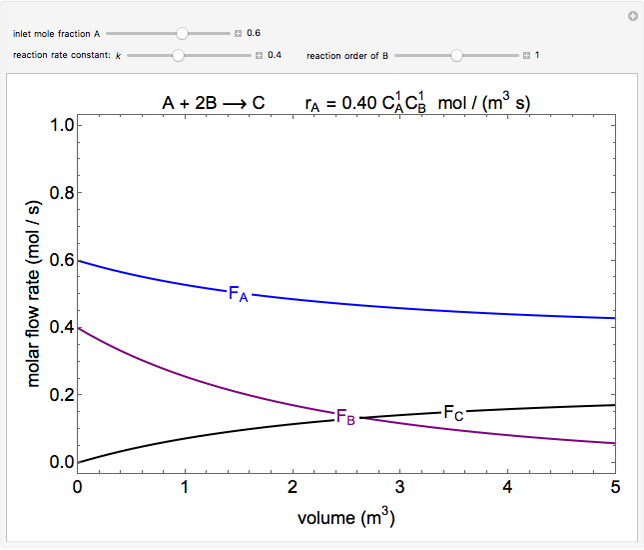
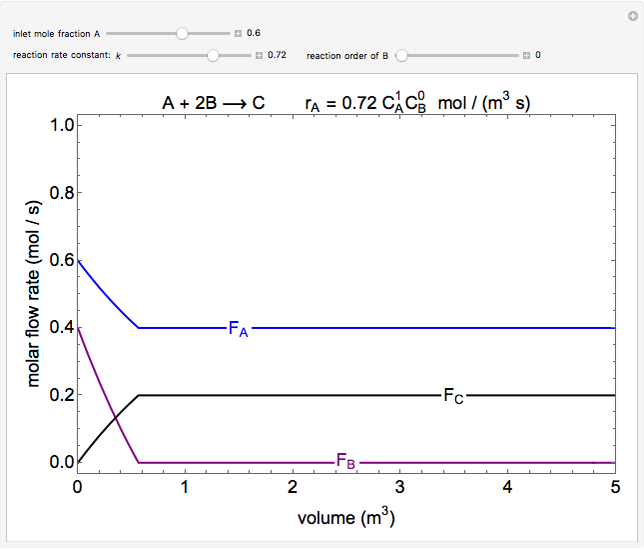
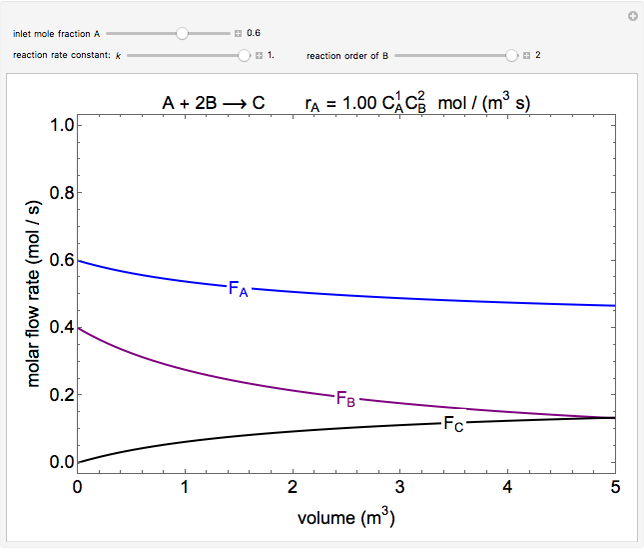
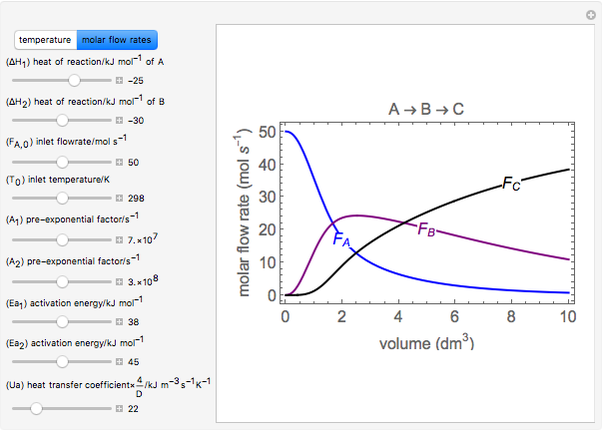
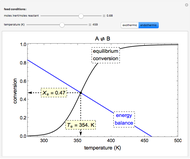
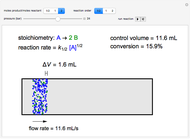
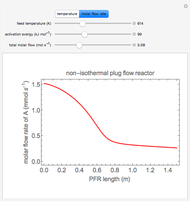
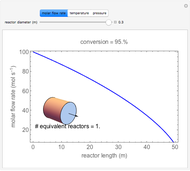
In this demonstration, the liquid-phase reaction A + 2B ⟶ C takes place in an isothermal plug flow reactor. Only A and B enter the reactor, and the user inputs the mole fraction of A in the feed. The reaction is first order in the concentration of A, but the user inputs the reaction order with respect to B and the value of the rate constant. When the reaction order is changed, the units of the rate constant changes but not is numerical value. The molar flow rates of A, B, and C (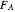 ,
, 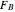 ,
, 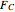 ) are plotted versus the cumulative reactor volume (the distance from the reactor inlet times the reactor cross-sectional area).
) are plotted versus the cumulative reactor volume (the distance from the reactor inlet times the reactor cross-sectional area).
Contributed by: Neil Hendren (February 2019)
Additional contributions by: John L. Falconer
Open content licensed under CC BY-NC-SA
Details
The constant-density liquid-phase reaction takes place in an isothermal plug flow reactor:
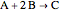 , with reaction rate
, with reaction rate 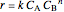 ,
,
where 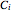 is the concentration of component
is the concentration of component 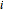 ,
,  is rate of reaction,
is rate of reaction,  is the order of reaction with respect to component
is the order of reaction with respect to component 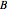 , and
, and  is the rate constant.
is the rate constant.
Mass balances on each component:
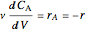 ,
,
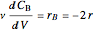 ,
,
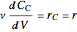 ,
,
where  is the cumulative volume of the plug flow reactor (i.e., the distance from the inlet times the cross-sectional area). Initial conditions
is the cumulative volume of the plug flow reactor (i.e., the distance from the inlet times the cross-sectional area). Initial conditions 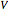 ,
, 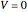 , and
, and 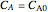 must be specified to fully solve the system of differential equations.
must be specified to fully solve the system of differential equations.
Snapshots
Permanent Citation