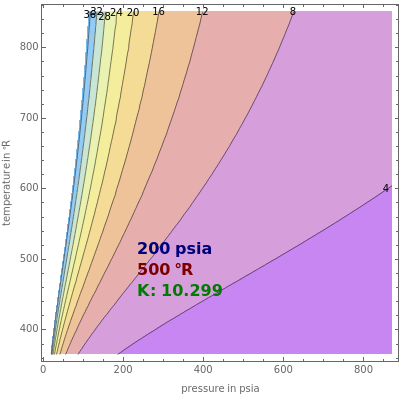
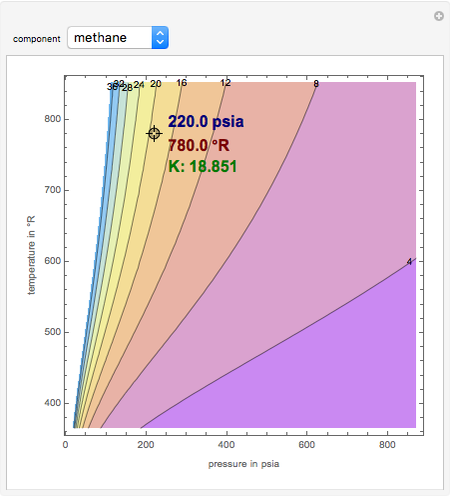
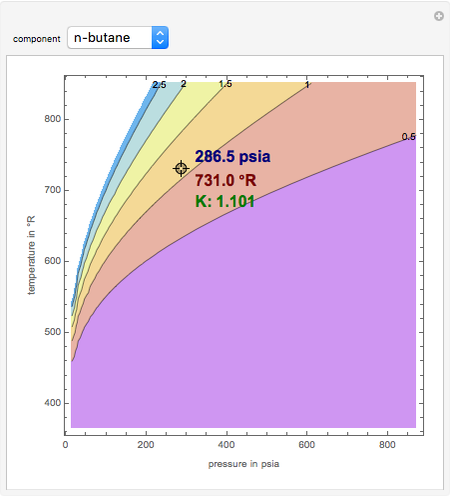
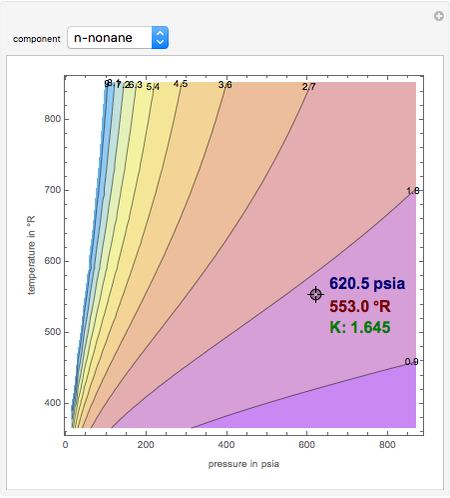
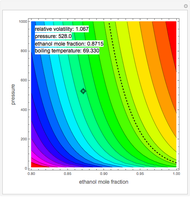
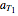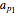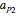K-value of Several Hydrocarbons versus Temperature and Pressure
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
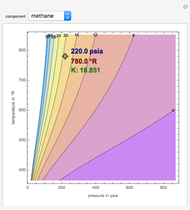
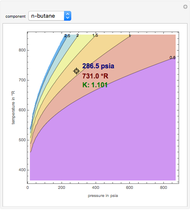
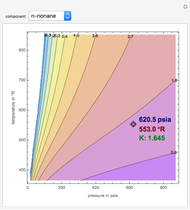
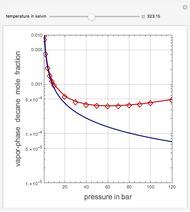
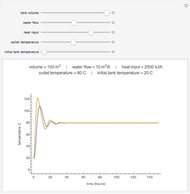
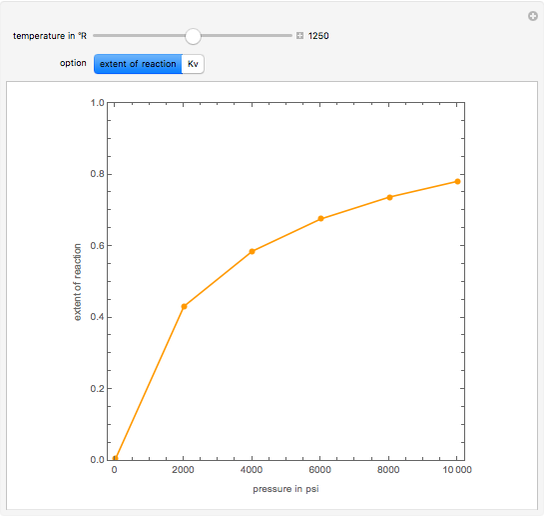
This Demonstration shows the contour plot of 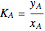 versus temperature expressed in °R and pressure expressed in psia, where
versus temperature expressed in °R and pressure expressed in psia, where 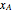 and
and  are the mole fractions of component
are the mole fractions of component 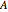 in the liquid and vapor phases, respectively. For many systems,
in the liquid and vapor phases, respectively. For many systems,  -values are approximately independent of composition and we have
-values are approximately independent of composition and we have 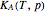 .
.
Contributed by: Housam Binous and Ahmed Bellagi (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
[1] M. L. McWilliams, "An Equation to Relate K-Factors to Pressure and Temperature," Chemical Engineering, 80(25), 1973 p. 138.
Permanent Citation