Liquid Chromatography with Linear Adsorption Equilibrium and Plate Model

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
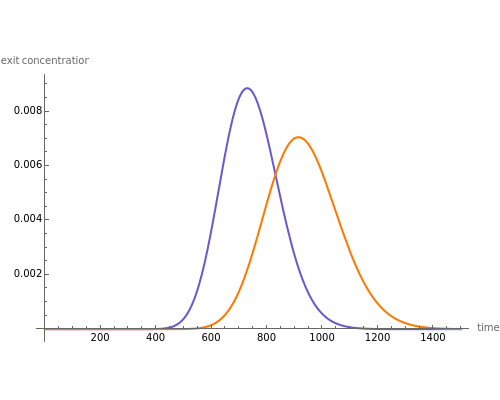
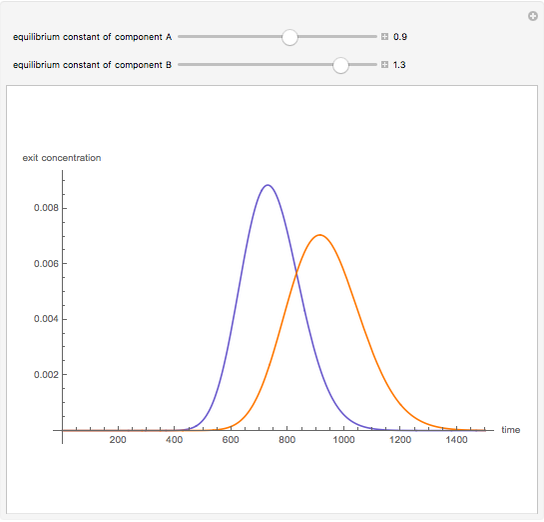
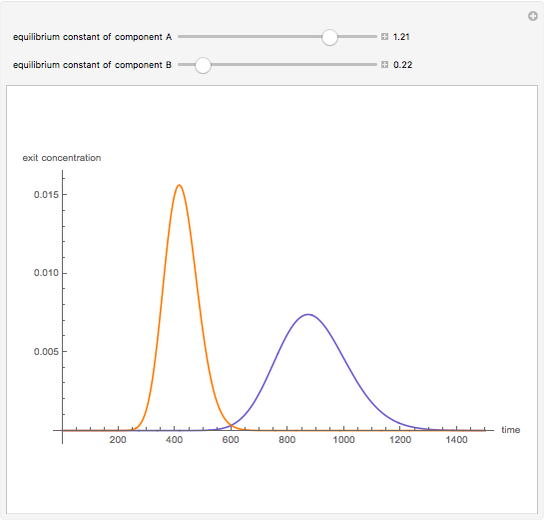
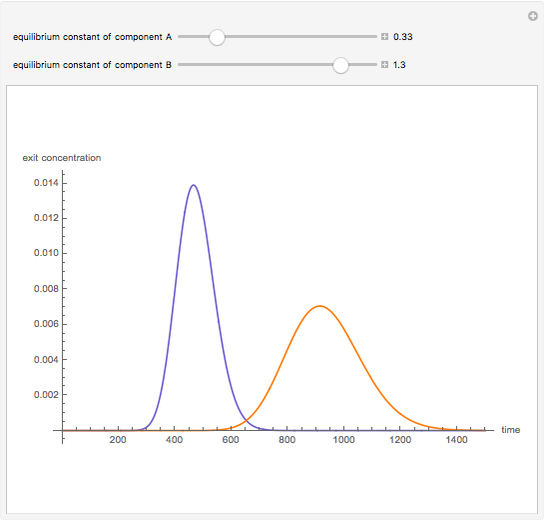
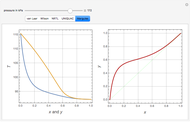
Liquid chromatography is a common technique in analytical chemistry used to identify and quantify chemical species in a mixture. The mixture is injected at the inlet of a chromatographic column and a liquid mobile phase (the solvent) allows their elution. The liquid chromatography technique is based on the difference in adsorption of the chemicals on a solid stationary phase or adsorbent. Hence, different chemical species will elute at different retention times.
[more]
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
J. Ingham, I. J. Dunn, E. Heinzle, and J. E. Prenosil, "CHROMPLATE - Stagewise Model for Chromatography Columns," Chemical Engineering Dynamics, 2nd ed., Weinheim, Germany: Wiley-VCH, 500 pp. 541–545.