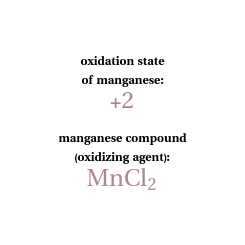Manganese Oxidation States

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
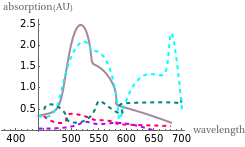
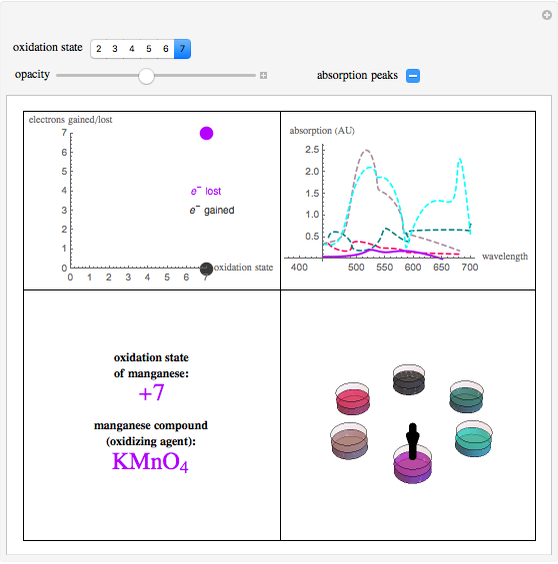
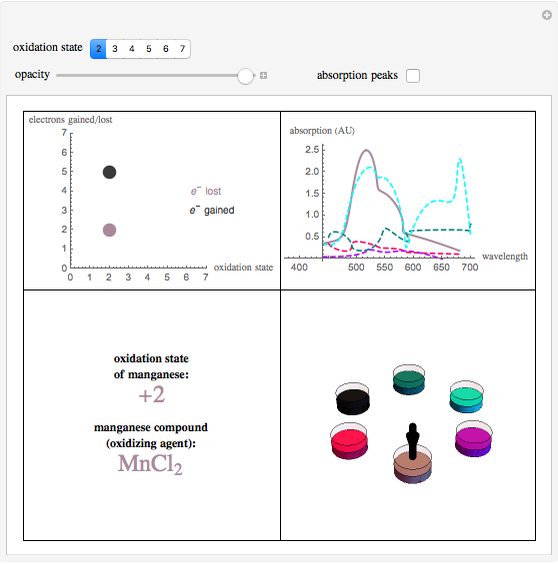
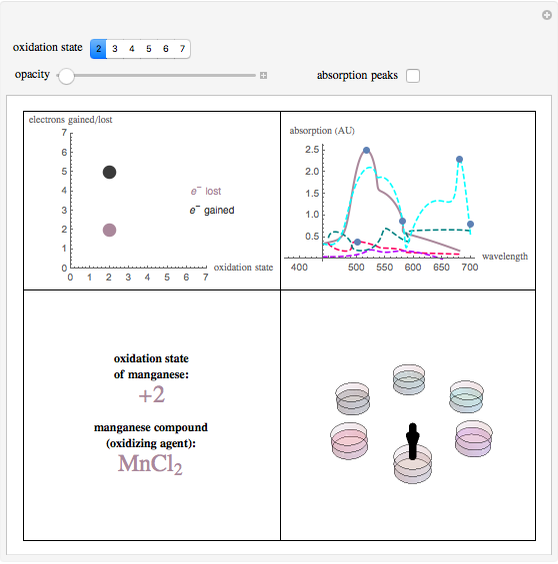
This Demonstration shows the colors and absorption spectra of the six most common oxidation states (2 to 7) of the element manganese. When you select an oxidation state, an arrow points to the “petri dish” containing an aqueous solution of a compound in this oxidation state, appropriately colored. Absorption spectra for these compounds are shown in the upper-right panel.
Contributed by: Chiranth Kishore and Emma Davies (June 2016)
Special thanks to the University of Illinois NetMath Program and the mathematics department at William Fremd High School.
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: this displays manganese in the +7 state, where it has lost its maximum number of electrons, with the petri dish opacity at 50%
Snapshot 2: this displays manganese in the +2 state with the petri dish opacity at 100%
Snapshot 3: this displays manganese in the +2 state with the petri dish opacity at 1%, the maximum absorption peaks are also shown
To best describe the color properties of each oxidation state, we found results [1–4] showing the absorption spectra of compounds in each respective oxidation state. By pinpointing the wavelength absorbed that resulted in the maximum absorbance and using a wavelength to RGB converter [5], the color absorbed was identified. The opacity slider reflects the relative concentration of solutions in these oxidation states. To find the color of the wavelength emitted (the actual color of the respective oxidation states), a color complement converter [6] was used from the aforementioned “color/wavelength absorbed”. This gives the most accurate color for each oxidation state (lower-right panel).
Additional contributions by Nathan Narasimhan and Connor Puritz for help with code efficiency and variable localization, Karl Craddock for confirming the accuracy of the chemistry, and Chris Grattoni for providing critique and instilling in us the love of math.
References
[1] C. E. Adeeyinwo, N. N. Okorie, and G. O. Idowu “Basic Calibration of UV/Visible Spectrophotometer,” International Journal of Science and Technology, 2(3), 2013 pp. 247–251.
[2] N. N. Okorie, “Determination of an Unknown Concentration of Potassium Manganate Using Colorimetry,” 2011 pp. 1–4. (Jun 6, 2016) .
[3] Center for Applied Isotope Studies, “Potassium: Method 258.1 (atomic absorption, direct aspiration),” Methods for Chemical Analysis of Water and Wastes, Washington, DC: United States Environmental Protection Agency, 1983 pp. 258.1-1–258.1-2.
[4] Scottish Schools Education Research Centre. “Oxidation States of Manganese.” (Jun 6, 2016) www.sserc.org.uk/index.php/chemistry-resources/chemistry-demonstrations/3692-oxidation-states-of-manganese.
[5] K. Spartalian. “Wavelength to RGB.” University of Vermont. (Jun 6, 2016) www.uvm.edu/~kspartal/Physlets/Lecturedemo/LambdaToRGB.html.
[6] Sessions College. “Color Wheel - Color Calculator.” (Jun 6, 2016) www.sessions.edu/color-calculator.
Permanent Citation
"Manganese Oxidation States"
http://demonstrations.wolfram.com/ManganeseOxidationStates/
Wolfram Demonstrations Project
Published: June 7 2016