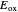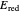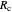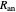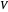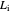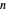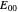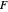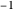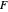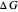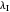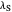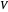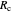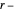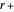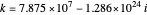Marcus Theory of Electron Transfer 3: Interactive Potential Energy Surfaces for Charge Separation

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
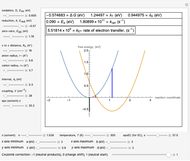
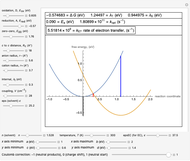
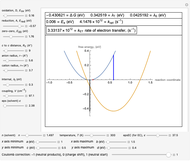
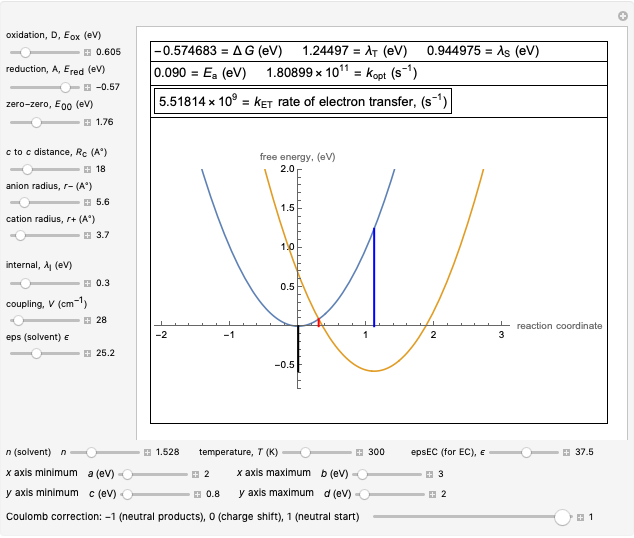
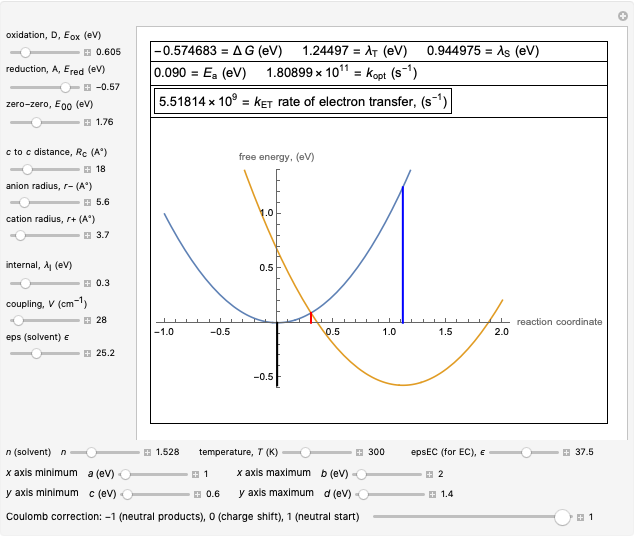
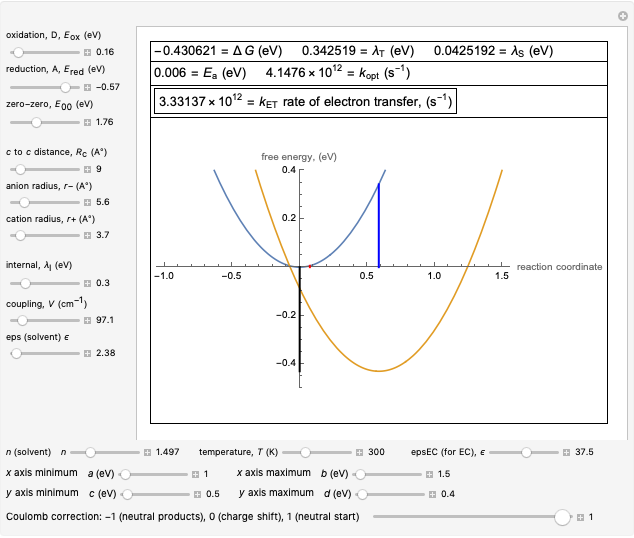
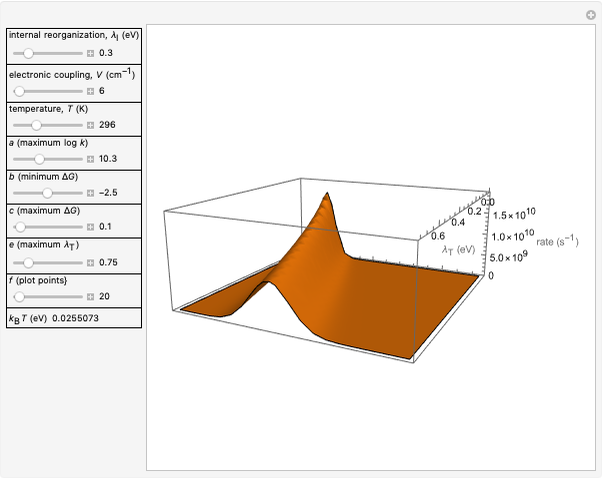
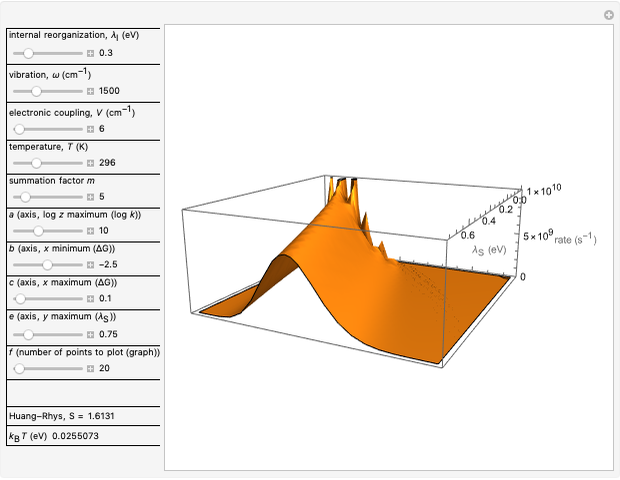
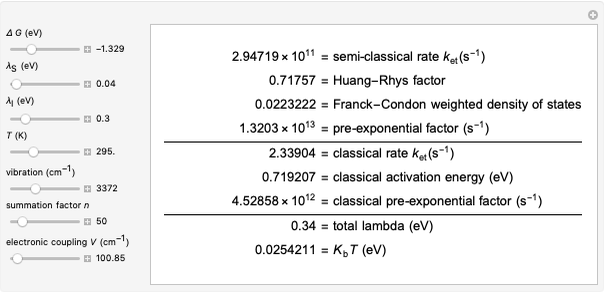
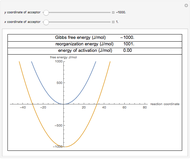
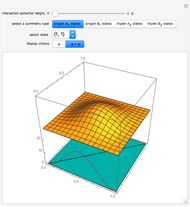
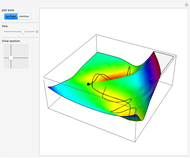
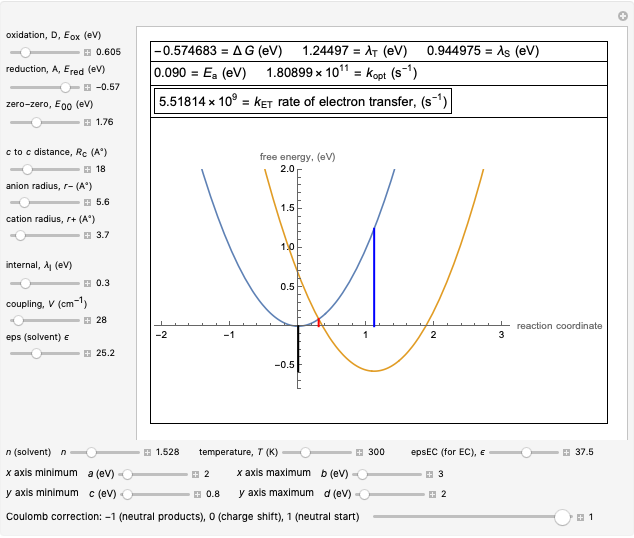
This Demonstration applies the classical Marcus equation to calculate and manipulate the rate of electron transfer (charge separation) by varying certain "molecular parameters". Eight molecular input parameters and four general input parameters are varied to calculate the Gibbs free energy of electron transfer 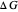 , the barrier
, the barrier 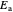 and the total reorganization energy
and the total reorganization energy 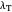 , as well as the rate of the charge separation
, as well as the rate of the charge separation 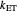 . The pre-exponential factor
. The pre-exponential factor 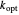 represents the rate in the absence of a barrier. You can use the sliders to observe the effects of the individual parameters on the rates and thermodynamic parameters.
represents the rate in the absence of a barrier. You can use the sliders to observe the effects of the individual parameters on the rates and thermodynamic parameters.
Contributed by: René M. Williams (August 2022)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: Data on charge separation for the compound C60[11]DMA in benzonitrile as solvent. Normal region.
Snapshot 2: Data on charge separation of the compound C60[3]TMPD in toluene as solvent. Optimal region.
Snapshot 3: Data on charge shift reaction of Closs and Miller in methyltetrahydrofuran as solvent. Inverted region, in the classical model.
Snapshot 4: Data on charge recombination of the compound C60[3]TMPD in toluene as solvent. Inverted region, in the classical model. Zero-zero energy adjusted to fit charge recombination.
References
[1] R. A. Marcus, "Electron Transfer Reactions in Chemistry: Theory and Experiment (Nobel Lecture)," Angewandte Chemie International Edition, 32(8), 1993 pp. 1111–1121. doi:10.1002/anie.199311113.
[2] S. Chaudhuri, S. Hedström, D. D. Méndez-Hernández, H. P. Hendrickson, K. A. Jung, J. Ho and V. S. Batista, "Electron Transfer Assisted by Vibronic Coupling from Multiple Modes," Journal of Chemical Theory and Computation, 13(12), 2017 pp. 6000–6009. doi:10.1021/acs.jctc.7b00513.
[3] P. F. Barbara, T. J. Meyer and M. A. Ratner, "Contemporary Issues in Electron Transfer Research," Journal of Physical Chemistry, 100(31), 1996 pp. 13148–13168. doi:10.1021/jp9605663.
[4] G. L. Closs and J. R. Miller, "Intramolecular Long-Distance Electron Transfer in Organic Molecules," Science, 240(4851), 1988 pp. 440–447. doi:10.1126/science.240.4851.440.
[5] P. Hudhomme and R. M. Williams, "Energy and Electron Transfer in Photo- and Electro-active Fullerene Dyads," Handbook of Carbon Nano Materials (F. D'Souza and K. M. Kadish, eds.), Hackensack, NJ: World Scientific, 2011 pp. 545–591. doi:10.1142/9789814327824_0017.
[6] R. M. Williams. "Introduction to Electron Transfer," (Jan 26, 2022) doi:10.13140/RG.2.2.16547.30244.
[7] R. M. Williams. Photoinduced Electron Transfer - The Classical Marcus Theory [Video]. (Jan 26, 2022) youtu.be/YFzeMMOvhl0.
[8] R. M. Williams. Photoinduced Electron Transfer - The Semi-classical Marcus-Levich-Jortner Theory [Video]. (Jan 26, 2022) youtu.be/GnPIbH6nM9o.
[9] R. M. Williams. University of Amsterdam. (Jan 21, 2022) www.uva.nl/en/profile/w/i/r.m.williams/r.m.williams.html.
[10] R. M. Williams. "22-Marcus-Mathematica-TESTDATA-Assignments." (Jan 26, 2022) doi.org/10.21942/uva.17912015.
Permanent Citation