Nonisothermal Degradation Kinetics

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
The orders of chemical and biochemical reactions, their rate constants, and how they vary with temperature are usually determined in a series of isothermal experiments on samples where diffusion and transient heat transfer considerations can be neglected. The kinetics of nonisothermal or dynamic reactions under such circumstances can be predicted from such data using rate equations with parameters varying with time. This Demonstration illustrates the principle by simulating concentration versus time relationships in degradation reactions or decay processes in which the temperature rises linearly, falls linearly, or oscillates sinusoidally, and also when the rate temperature dependence of the rate constant is characterized by an Arrhenius or exponential model.
Contributed by: Mark D. Normand and Micha Peleg (August 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
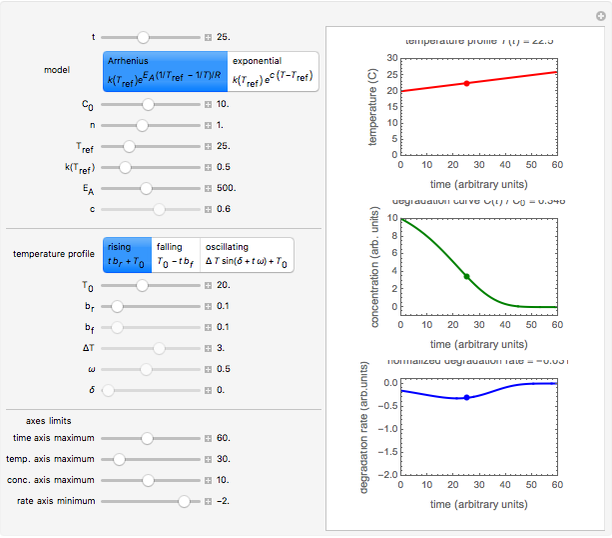
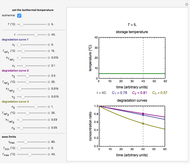
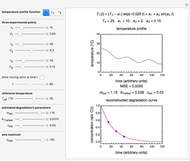
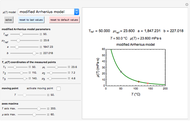
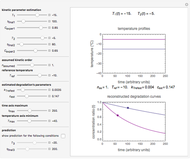
Snapshot 1: degradation curve in a reaction following the Arrhenius and first-order ( ) kinetics equations with a linearly rising temperature
) kinetics equations with a linearly rising temperature
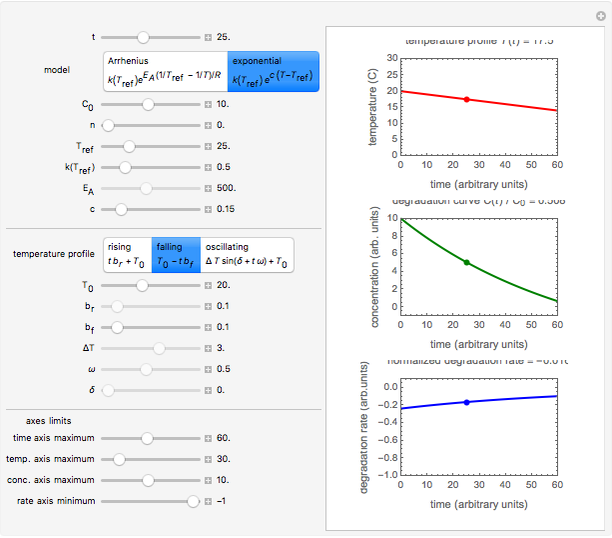
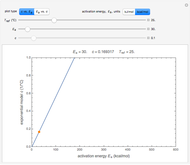
Snapshot 2: degradation curve in a reaction following the exponential model and zero-order kinetics ( ) with a linearly falling temperature
) with a linearly falling temperature
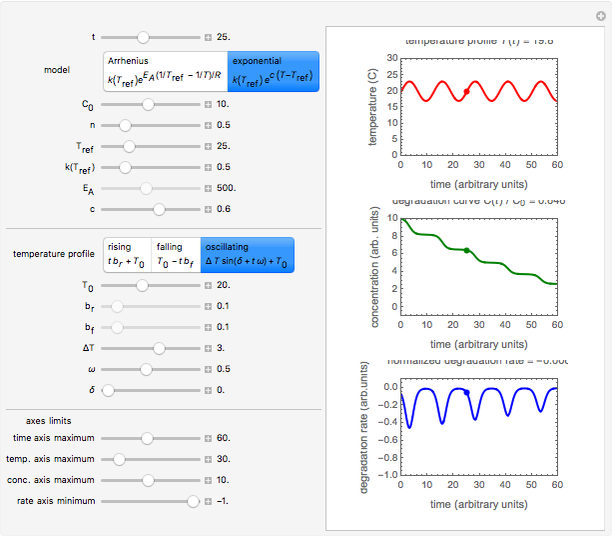
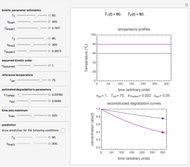
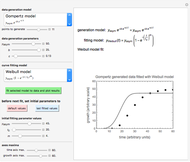
Snapshot 3: degradation curve in a reaction following the exponential model and half-order kinetics (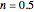 ) with a sinusoidally oscillating temperature
) with a sinusoidally oscillating temperature
The kinetic order of a degradation reaction or decay process is defined as the exponent  in the static (isothermal) rate equation
in the static (isothermal) rate equation 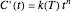 with the boundary condition
with the boundary condition 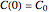 in the equation representing the momentary concentration at time
in the equation representing the momentary concentration at time  and the initial concentration, respectively, and
and the initial concentration, respectively, and 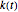 , the temperature-dependent rate constant having units consistent with
, the temperature-dependent rate constant having units consistent with  . When the temperature varies,
. When the temperature varies, 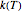 becomes
becomes 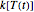 and the rate equation's solution, which is more often numerical than analytical, depends on the particulars of the temperature history
and the rate equation's solution, which is more often numerical than analytical, depends on the particulars of the temperature history 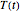 . This Demonstration generates
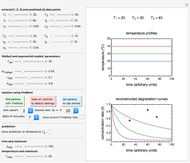
. This Demonstration generates 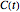 curves for linearly rising, linearly falling, and oscillating (sinusoidal) temperature histories whose parameters you can enter and vary with sliders. You can also enter the order
curves for linearly rising, linearly falling, and oscillating (sinusoidal) temperature histories whose parameters you can enter and vary with sliders. You can also enter the order  of the reaction or process in the range from 0. to 2.5 with a slider. Although the model equation for
of the reaction or process in the range from 0. to 2.5 with a slider. Although the model equation for  applies to
applies to  , we treat this scenario as a special case because of its simplicity.
, we treat this scenario as a special case because of its simplicity.
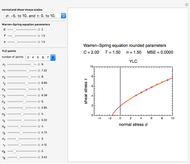
The temperature dependence of the rate constant 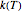 is described by either the Arrhenius equation
is described by either the Arrhenius equation 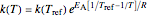 , where
, where 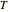 and
and 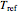 are the actual and reference temperatures, respectively, in K,
are the actual and reference temperatures, respectively, in K,  is the energy of activation, and
is the energy of activation, and 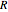 the universal gas constant, or the simpler exponential model [1]
the universal gas constant, or the simpler exponential model [1] 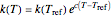 , where
, where 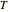 and
and 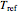 are in
are in  and
and  is a constant having
is a constant having 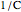 units. One can show that the two models have a considerable range of overlap around the reference temperature
units. One can show that the two models have a considerable range of overlap around the reference temperature 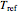 and that
and that 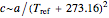 or
or  , where
, where 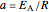 [1].
[1].
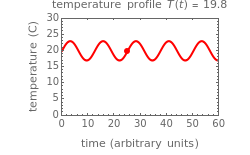
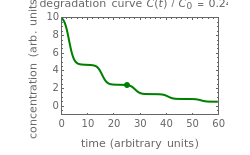
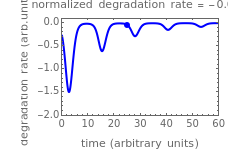
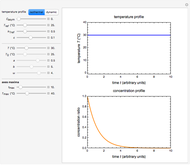
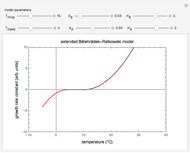
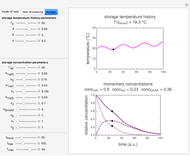
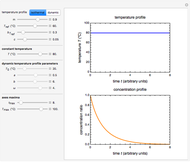
This Demonstration shows the temperature history (top) and the corresponding concentration and concentration decay rate curves (middle and bottom) with the momentary numerical values of 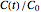 and the concentration decay rate at a time
and the concentration decay rate at a time  (which you can choose with a slider) shown as a moving dot on the three plots.
(which you can choose with a slider) shown as a moving dot on the three plots.
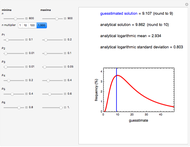
The purpose of this Demonstration is to illustrate how a reaction's kinetic order affects the concentration decay curve under nonisothermal conditions. Therefore, not all the decay and decay rate curves that it can produce necessarily represent an actual physical disintegration or degradation process.
Reference
[1] M. Peleg, M. D. Normand, and M. G. Corradini, "The Arrhenius Equation Revisited," Critical Reviews in Foods Science and Nutrition, 52, 2012 pp. 830–851.
Permanent Citation