Operation of an Ethane Steam Cracker

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
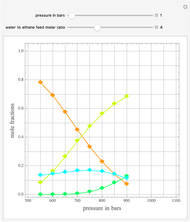
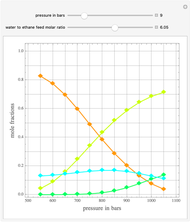
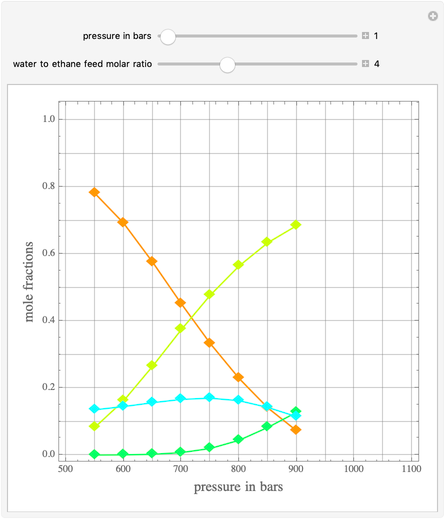
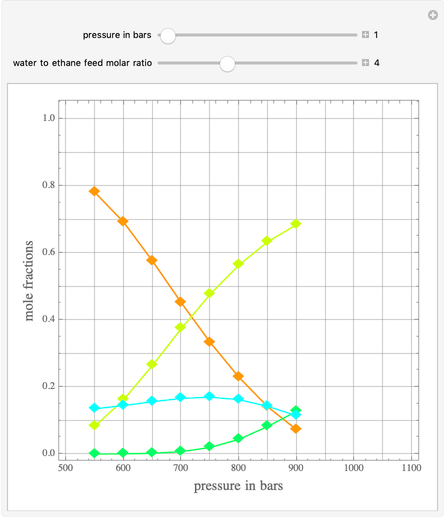
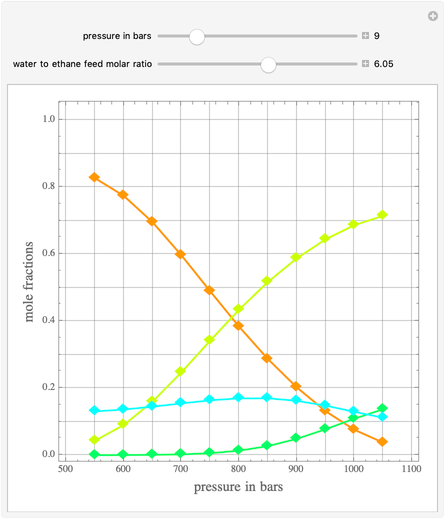
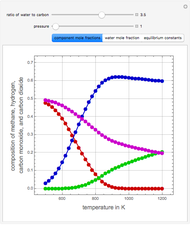
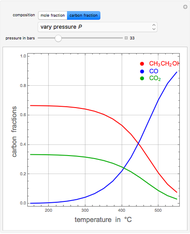
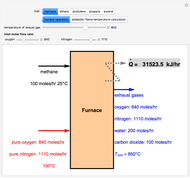
Ethane and steam are fed to a steam cracker at a user-set total pressure (in bars). The water/ethane molar ratio  is also set by the user. This Demonstration estimates the equilibrium distribution of products (methane, ethylene, acetylene, carbon dioxide, carbon monoxide, oxygen, hydrogen, water, and ethane) for temperatures ranging from 550 K to 1100 K. The Gibbs free energy for the mixture is minimized subject to elemental mass balance constraints, which are easily obtained. It turns out that the equilibrium mole fractions of ethane, ethylene, acetylene, and oxygen are negligible for the selected range of
is also set by the user. This Demonstration estimates the equilibrium distribution of products (methane, ethylene, acetylene, carbon dioxide, carbon monoxide, oxygen, hydrogen, water, and ethane) for temperatures ranging from 550 K to 1100 K. The Gibbs free energy for the mixture is minimized subject to elemental mass balance constraints, which are easily obtained. It turns out that the equilibrium mole fractions of ethane, ethylene, acetylene, and oxygen are negligible for the selected range of  and
and  . Thus, only methane, hydrogen, carbon monoxide, and carbon dioxide need be plotted, in orange, yellow, green, and cyan, respectively.
. Thus, only methane, hydrogen, carbon monoxide, and carbon dioxide need be plotted, in orange, yellow, green, and cyan, respectively.
Contributed by: Housam Binous, Ahmed Bellagi, Brian G. Higgins, Ahmed Aheed, and Mohammad Mozahar Hossain (December 2014)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] I. Barin and G. Platzki, Thermochemical Data of Pure Substances, 3rd ed., New York: VCH Publishers, Inc., 1995.
[2] S. I. Sandler, Chemical and Engineering Thermodynamics, 3rd ed., New York: John Wiley & Sons, 1999.
Permanent Citation