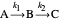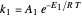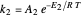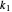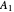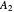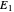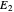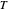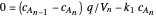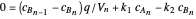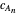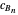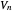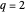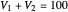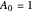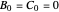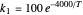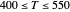Optimizing Temperatures and Volumes for Maximum Conversion to an Intermediate Reactant in a Chain of Two Continuous Stirred-Tank Reactors
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
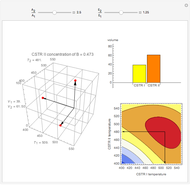
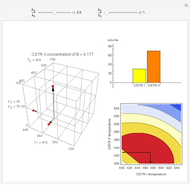
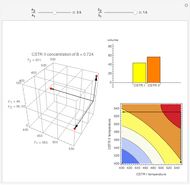
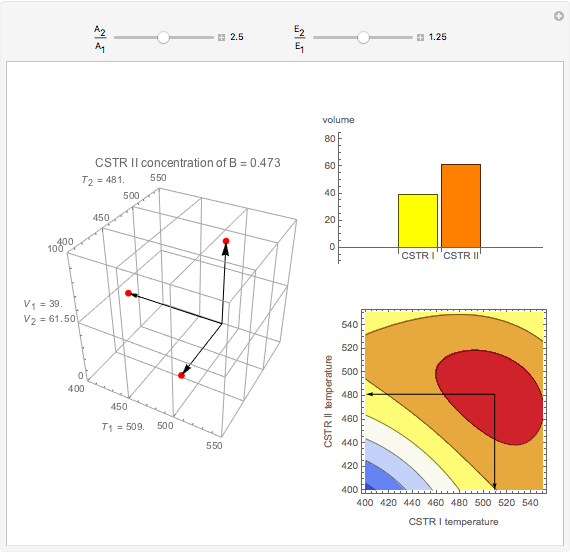
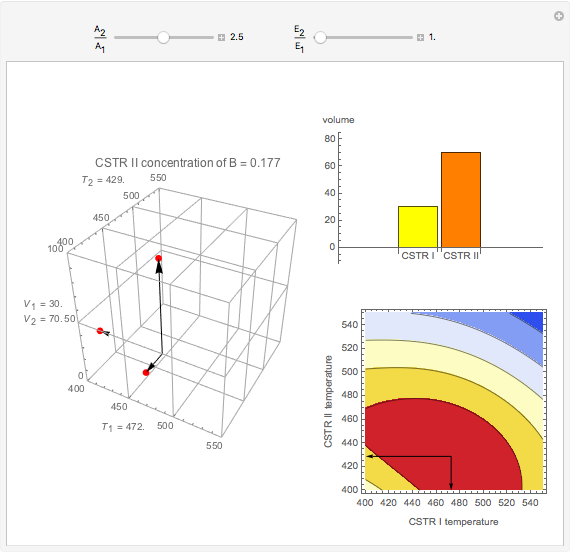
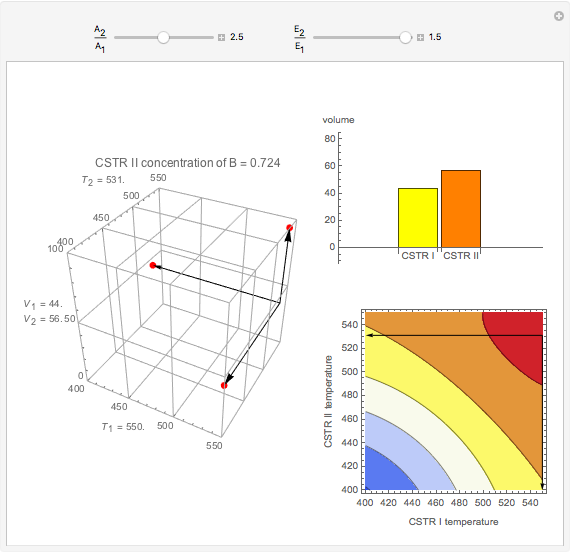
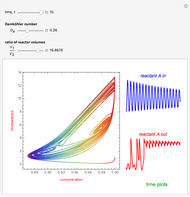
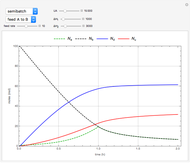
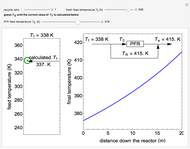
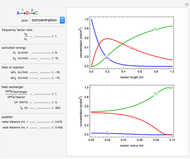
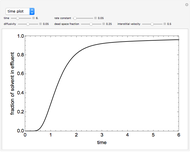
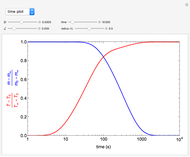
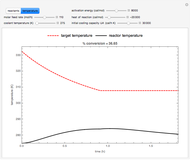
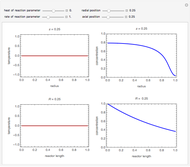
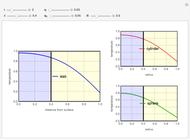
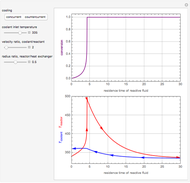
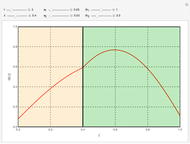
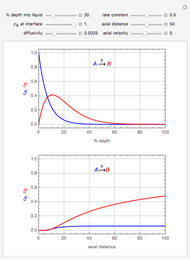
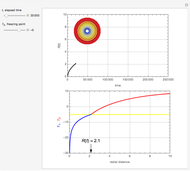
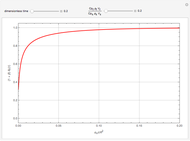
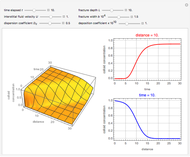
This Demonstration examines a two-stage continuous stirred-tank reactor (CSTR). Conversion to an intermediate product of two consecutive chemical reactions can be maximized by operating the system at the optimal volume and temperature for each reactor.
[more]
Contributed by: Clay Gruesbeck (February 2014)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] Wikipedia. "Continuous Reactor." (Aug 21, 2012) en.wikipedia.org/wiki/Continuous_reactor.
Permanent Citation