Oxidation States of Carbon

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
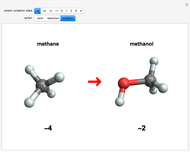
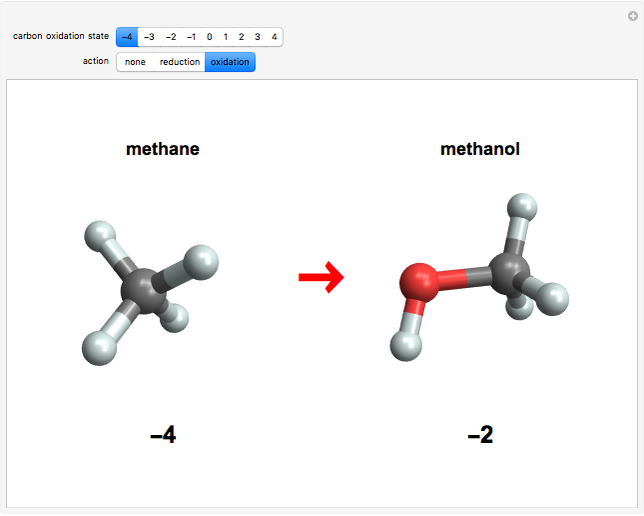
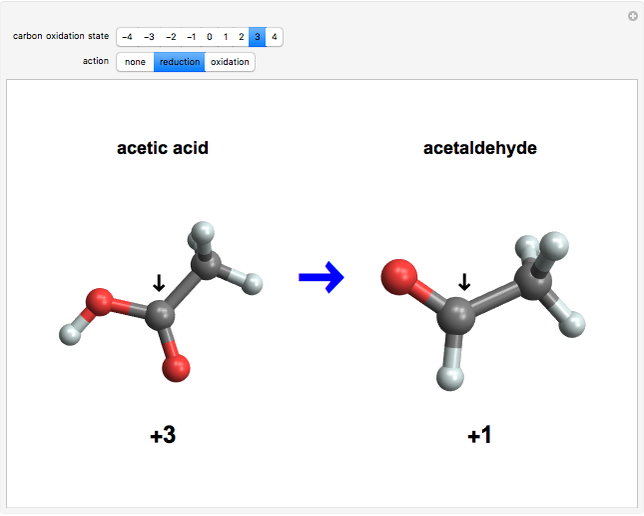
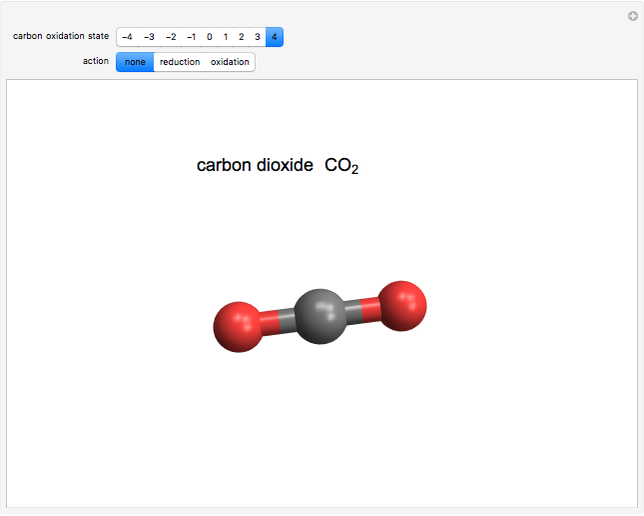
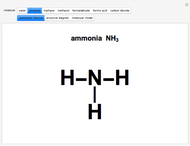
This Demonstration enumerates the possible oxidation states of carbon in a number of compounds containing one or two carbon atoms (colored gray) bonded to hydrogen atoms (white) and oxygen atoms (red). The concept of oxidation state is, to a large extent, a formal construct. It can be defined as the difference between the expected number of valence electrons for a neutral atom of an element and the number of electrons that are actually associated with that atom in a conventional Lewis structure. Carbon atoms can exist in nine different oxidation states, running from  to
to  . For neutral molecules, the oxidation number of each carbon atom can usually be found by assigning oxidation numbers of
. For neutral molecules, the oxidation number of each carbon atom can usually be found by assigning oxidation numbers of  and
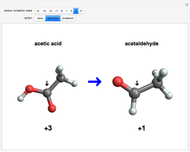
and  , respectively, to bonded hydrogen and oxygen atoms, then assigning the carbon values so that the total sums to zero. It is possible for different carbon atoms in the same molecule to belong in different oxidation states, for example, in ethanol and acetaldehyde. The alkane, alcohol, carbonyl, and carboxylic acid functional groups all appear in this series of compounds.
, respectively, to bonded hydrogen and oxygen atoms, then assigning the carbon values so that the total sums to zero. It is possible for different carbon atoms in the same molecule to belong in different oxidation states, for example, in ethanol and acetaldehyde. The alkane, alcohol, carbonyl, and carboxylic acid functional groups all appear in this series of compounds.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference: Oxidation Stateon Wikipedia.
Permanent Citation
"Oxidation States of Carbon"
http://demonstrations.wolfram.com/OxidationStatesOfCarbon/
Wolfram Demonstrations Project
Published: March 7 2011