Partial Molar Enthalpy and Entropy Quiz

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
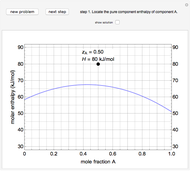
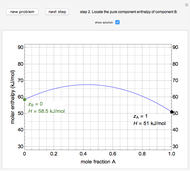
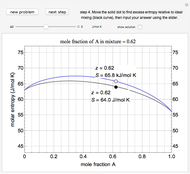
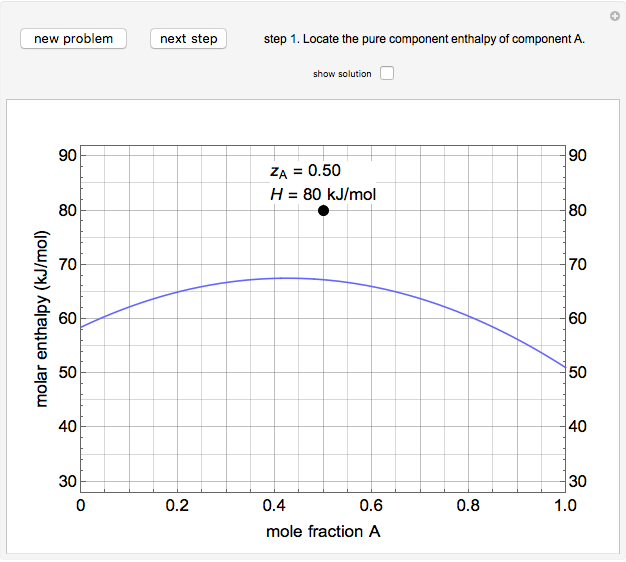
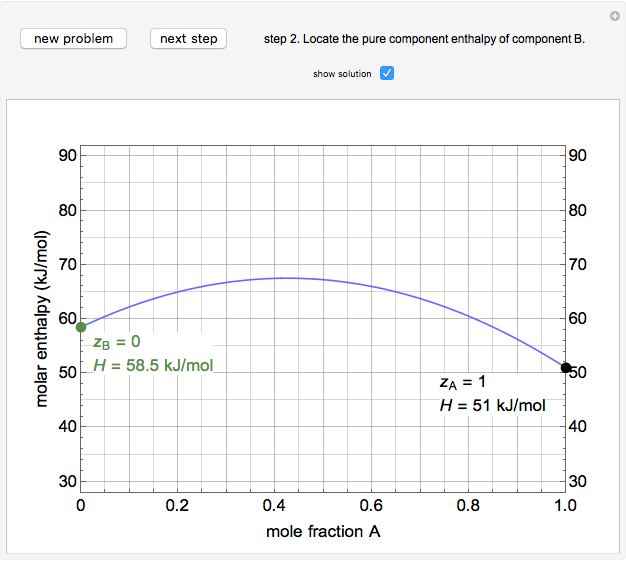
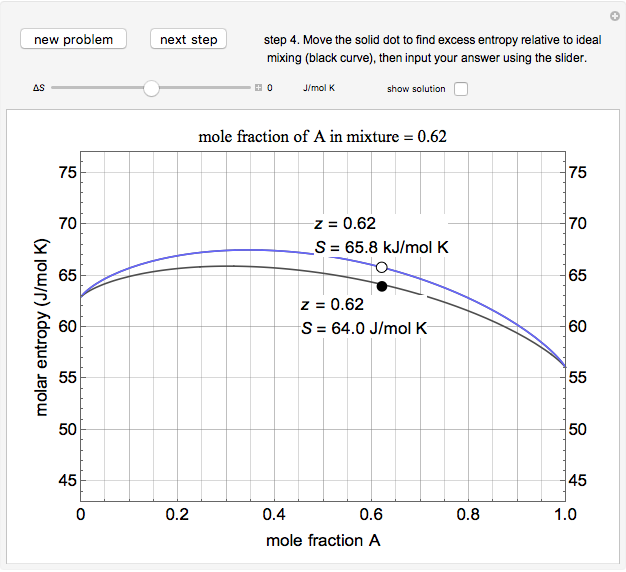
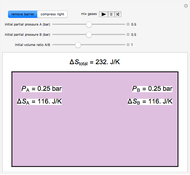
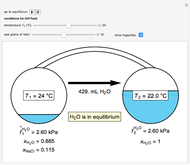
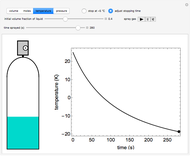
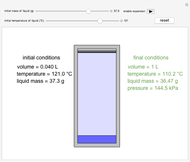
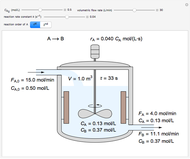
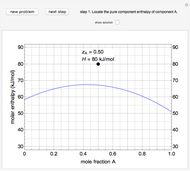
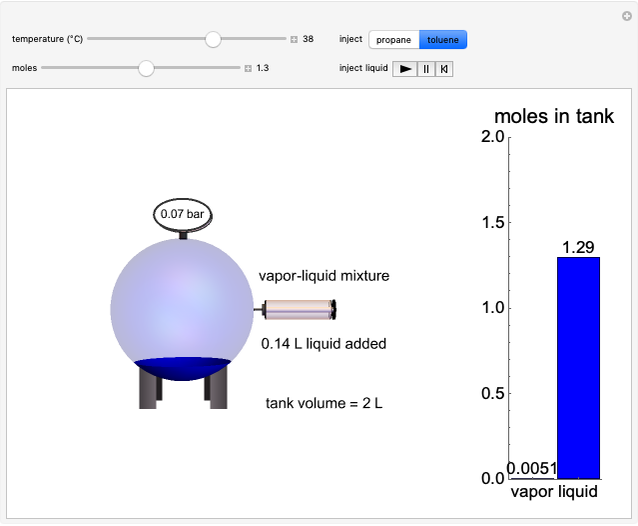
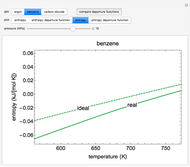
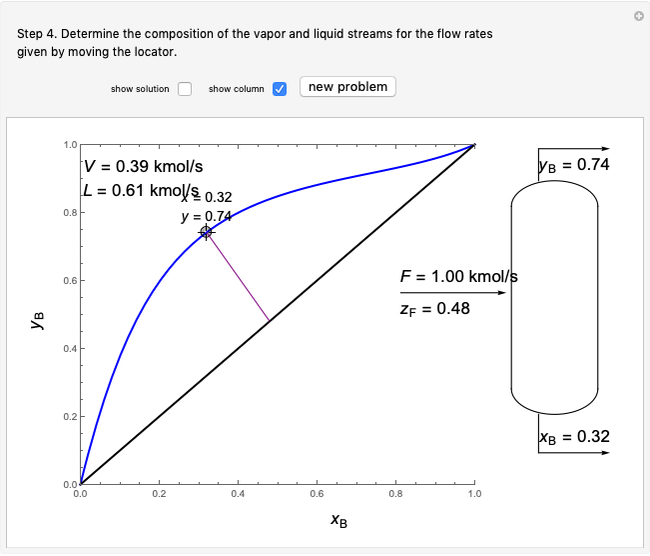
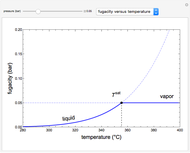
An interactive step-by-step procedure requires the user to identify pure component and mixture enthalpies or entropies in a binary solution. The user determines the excess enthalpy or entropy (differences from an ideal solution). For enthalpies, the user then calculates the temperature change for adiabatic mixing. The partial molar enthalpies and entropies are determined from lines tangent to the enthalpy or entropy curves. Selecting "new problem" at any time resets to step 1 with different numerical values and either an enthalpy or entropy plot randomly selected.
Contributed by: Neil Hendren (February 2019)
With additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Details
The molar enthalpy  is given by:
is given by:
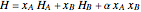 , (1)
, (1)
where 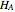 and
and 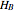 are the pure-component enthalpies (kJ/mol),
are the pure-component enthalpies (kJ/mol), 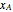 and
and 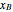 are the mole fractions of
are the mole fractions of 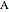 and
and 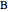 and
and  is a non-ideal parameter, which can be either a positive or negative value. The excess enthalpy
is a non-ideal parameter, which can be either a positive or negative value. The excess enthalpy 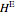 is:
is:
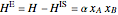 , (2)
, (2)
where 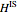 is the enthalpy of an ideal solution.
is the enthalpy of an ideal solution.
The change in temperature for adiabatic mixing 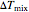 is a function of the excess enthalpy
is a function of the excess enthalpy 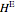 and the heat capacity of the solution (
and the heat capacity of the solution (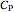 ).
).
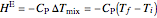 , (3)
, (3)
where 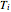 and
and 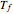 represent the initial and final temperatures of the solution.
represent the initial and final temperatures of the solution.
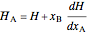 , (4)
, (4)
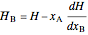 . (5)
. (5)
The molar entropy 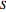 of an ideal solution is:
of an ideal solution is:
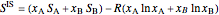 . (6)
. (6)
The excess entropy 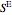 can be derived from excess Gibbs energy of mixing
can be derived from excess Gibbs energy of mixing 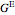 and an interaction parameter for the two components
and an interaction parameter for the two components 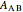 .
.
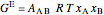 , (7)
, (7)
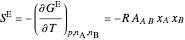 . (8)
. (8)
The partial molar entropies can be found from interactions of a line tangent to the 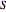 versus
versus 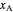 curve using equations analogous to equations (4) and (5).
curve using equations analogous to equations (4) and (5).
Snapshots
Permanent Citation