Peng-Robinson Equation of State for Mixtures

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
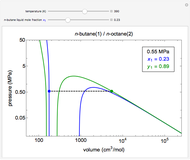
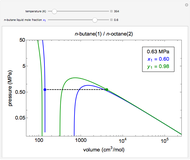
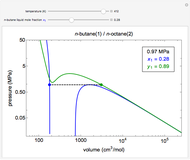
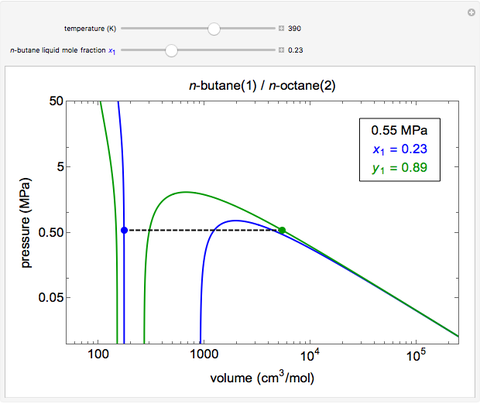
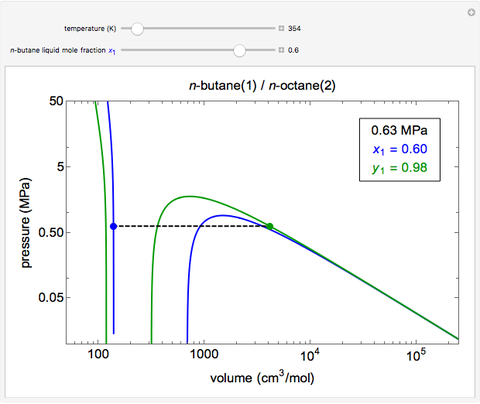
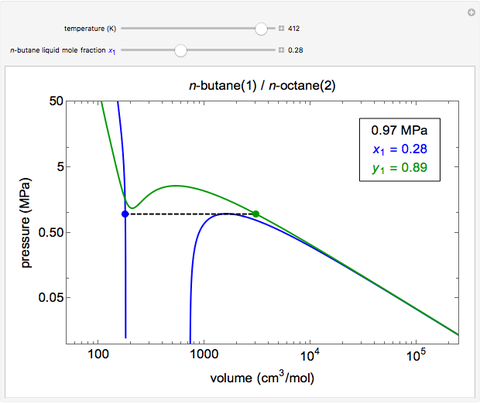
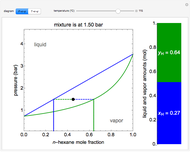
This Demonstration uses the Peng–Robinson equation of state for mixtures to plot isotherms for  -butane(1)/
-butane(1)/ -octane(2) mixtures on a log pressure versus log volume graph. Select the temperature with a slider. Selecting the mole fraction
-octane(2) mixtures on a log pressure versus log volume graph. Select the temperature with a slider. Selecting the mole fraction 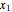 of the liquid yields the isotherm for the liquid in blue. The green isotherm is for the vapor phase (mole fraction
of the liquid yields the isotherm for the liquid in blue. The green isotherm is for the vapor phase (mole fraction 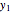 ) that is in equilibrium with the liquid; the values of
) that is in equilibrium with the liquid; the values of 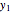 and pressure are determined from Raoult's law and are displayed in the upper-right corner. The dashed black line connects the blue isotherm to the green isotherm at the VLE pressure.
and pressure are determined from Raoult's law and are displayed in the upper-right corner. The dashed black line connects the blue isotherm to the green isotherm at the VLE pressure.
Contributed by: Rachael L. Baumann (February 2016)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The Peng–Robinson equation of state for mixtures is used to plot pressure 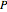 versus volume
versus volume 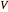 on a log-log graph:
on a log-log graph:
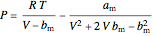 ,
,
where 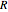 is the gas constant (
is the gas constant (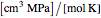 ),
), 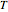 is temperature (K),
is temperature (K), 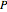 is in MPa and
is in MPa and 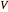 is in
is in 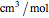 .
.
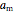 is the attraction parameter and
is the attraction parameter and 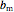 is the repulsion parameter for the mixture:
is the repulsion parameter for the mixture:
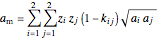 ,
,
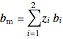 ,
,
where  is the component mole fraction,
is the component mole fraction, 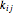 is the binary interaction parameter and
is the binary interaction parameter and  and
and  are the attraction and repulsion parameters for a pure component.
are the attraction and repulsion parameters for a pure component.
The binary interaction parameter can be calculated [1]:
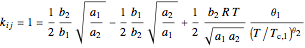 ,
,
for an alkane/alkane mixture 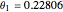 and
and 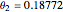 .
.
The attraction and repulsion parameters for a pure component are:
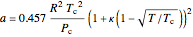 ,
,
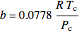 ,
,
where 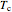 is the critical temperature (K) and
is the critical temperature (K) and 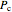 is the critical pressure (MPa).
is the critical pressure (MPa).
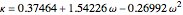 ,
,
where  is a simplification term and
is a simplification term and  is the acentric factor.
is the acentric factor.
Raoult's law is used to calculate the pressure of the mixture at VLE:
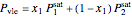 ,
,
and 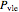 is used to determine the vapor mole fraction:
is used to determine the vapor mole fraction:
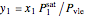 ,
,
where 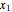 and
and 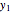 are the liquid and vapor mole fractions of hexane, and
are the liquid and vapor mole fractions of hexane, and 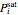 is the saturation pressure that is calculated using the Antoine equation:
is the saturation pressure that is calculated using the Antoine equation:
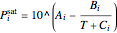 ,
,
where 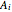 ,
, 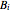 and
and 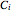 are Antoine constants.
are Antoine constants.
The screencast video at [2] explains how to use this Demonstration.
References
[1] A. O. Elnabawy, S. K. Fateen and M. M. Khalil, "Semi-empirical Correlation for Binary Interaction Parameters of the Peng–Robinson Equation of State with the van der Waals Mixing Rules for the Prediction of High-Pressure Vapor–Liquid Equilibrium," Journal of Advanced Research, 4(2), 2013 pp. 137–145. doi:10.1016/j.jare.2012.03.004.
[2] Peng-Robinson Equation of State for Mixtures [Video]. (Sep 1 2016) www.colorado.edu/learncheme/thermodynamics/PengRobinsonEOSMixtures.html.
Permanent Citation