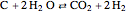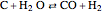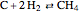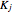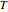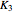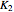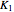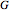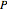Production of Syngas Using Coal-Steam Reactions
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
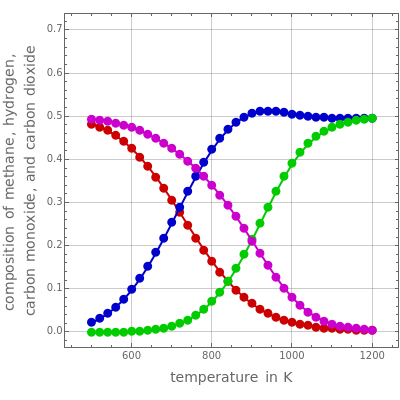
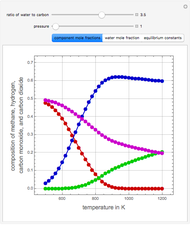
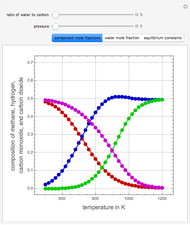
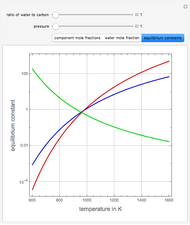
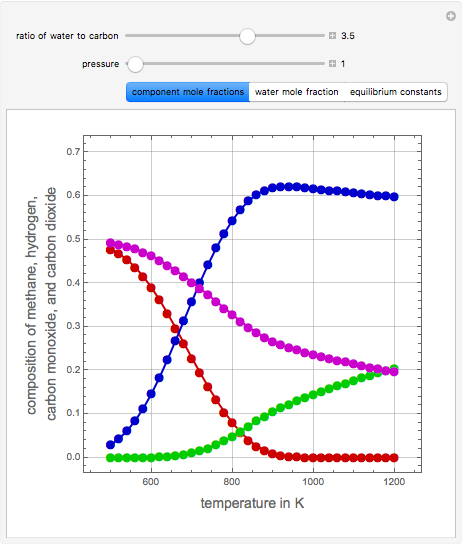
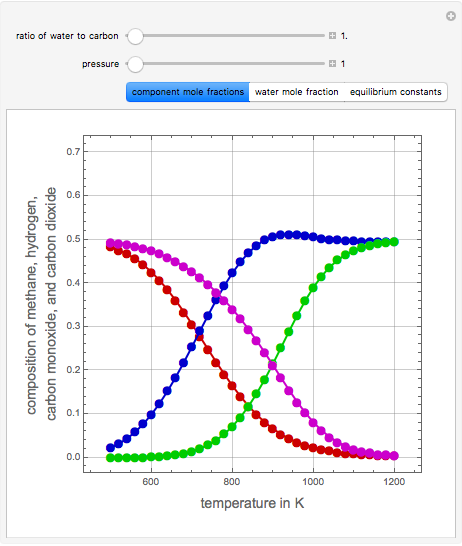
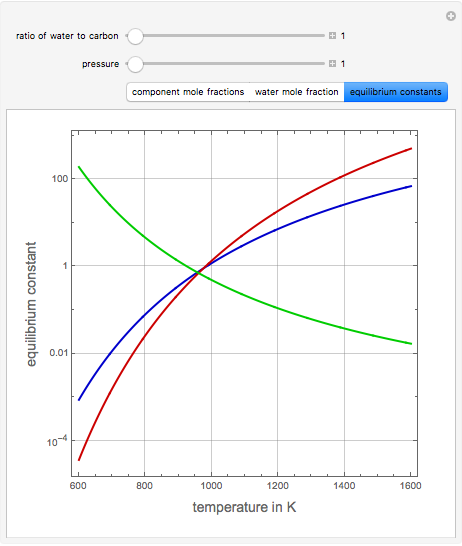
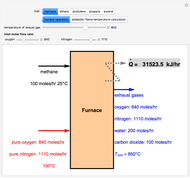
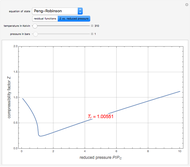
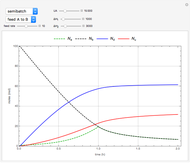
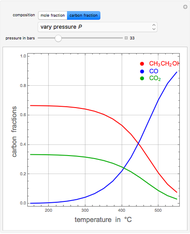
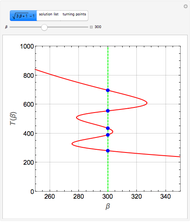
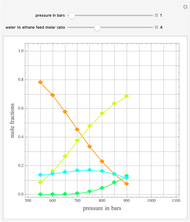
Consider the following three equilibrium reactions involving coal and steam which take place simultaneously in the syngas production process:
[more]
Contributed by: Housam Binous, Ahmed Bellagi, Brian G. Higgins, Ahmed Aheed, and Mohammad Mozahar Hossain (January 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] S. I. Sandler, Chemical and Engineering Thermodynamics, 3rd ed., New York: John Wiley & Sons, 1999.
Permanent Citation