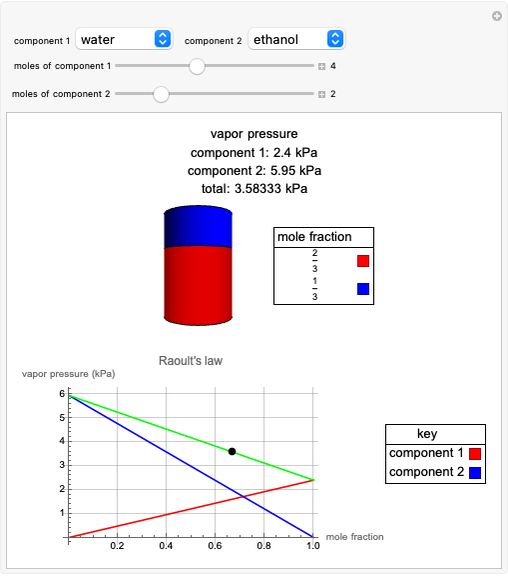
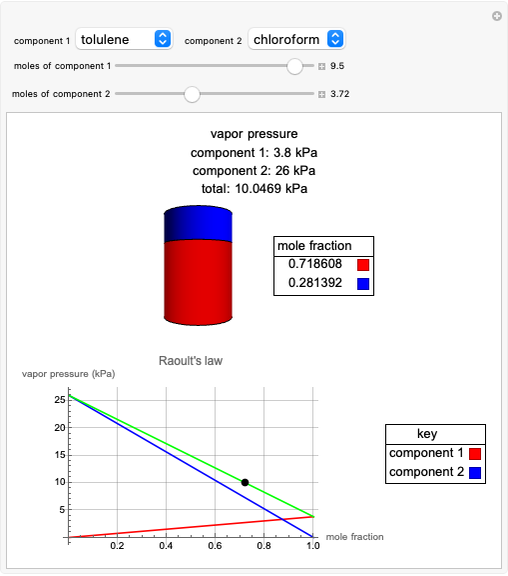
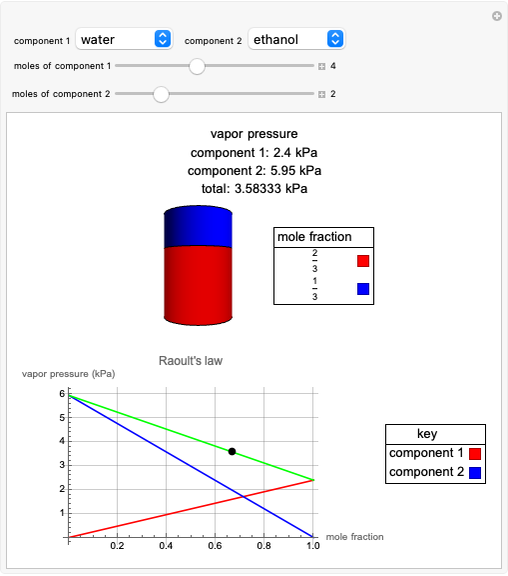
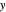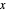Raoult's Law for Ideal Solutions
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
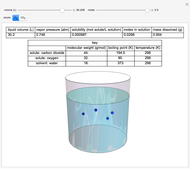
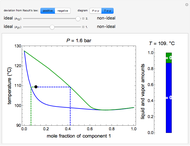
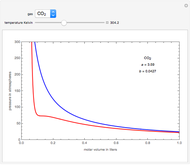
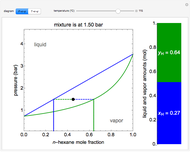
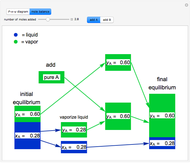
Raoult's law gives the vapor pressure of an ideal solution of two components at a constant temperature.
[more]
Contributed by: Camila Amarista and Megan Tafoya (May 8)
Open content licensed under CC BY-NC-SA
Details
Snapshots
Permanent Citation