Residue Curve Map for a Mixture of Water, Ethanol, and Ethylene Glycol

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
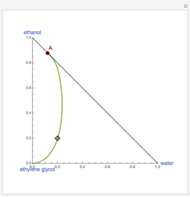
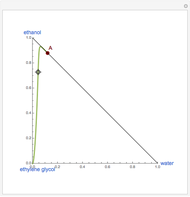
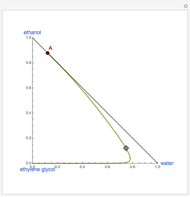
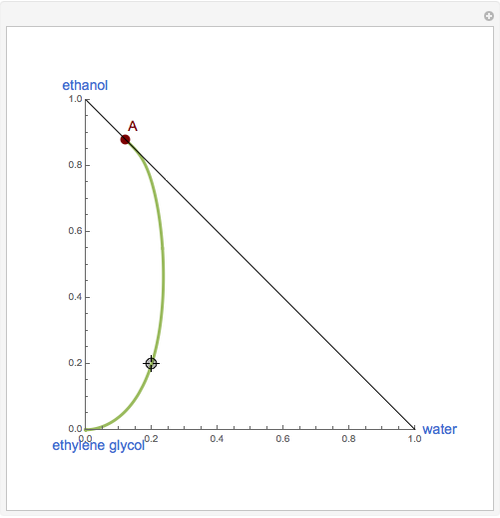
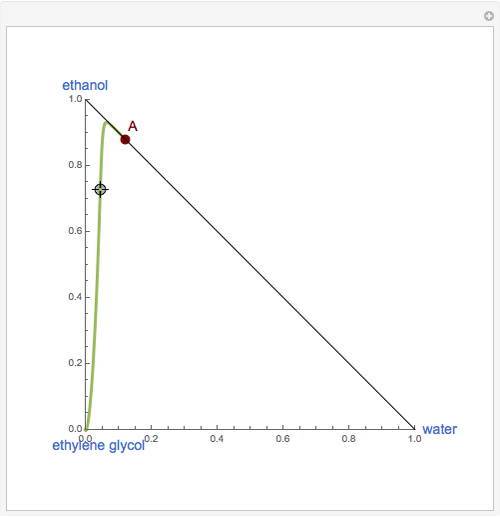
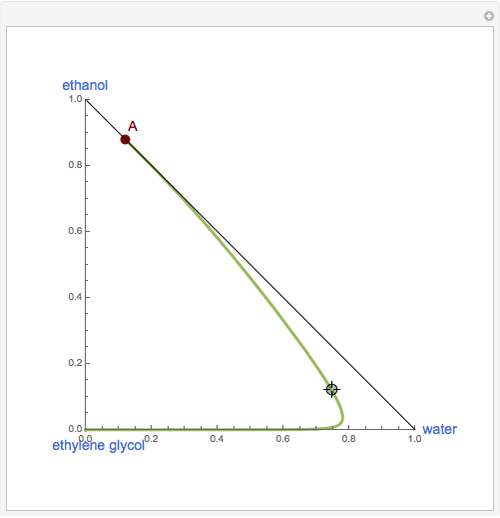
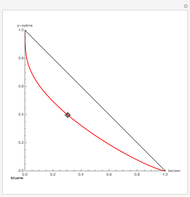
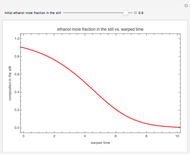
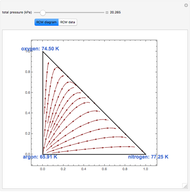
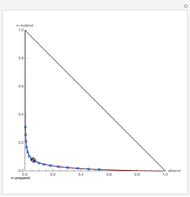
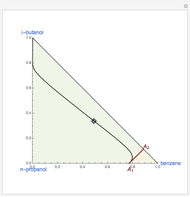
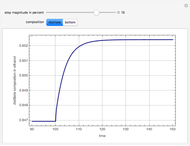
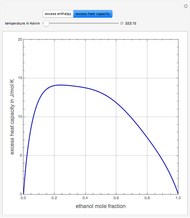
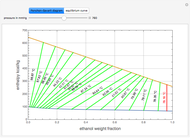
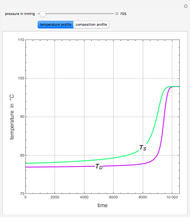
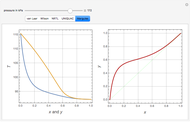
This Demonstration plots the residue curve of a non-ideal ternary mixture composed of water, ethanol, and ethylene glycol. Deviation from ideal behavior in the liquid phase is taken into account using the Wilson model in order to compute the activity coefficients. Total pressure is equal to 101.325 kPa. This mixture presents one azeotrope between water and ethanol, marked in the ternary diagram by  (88.08% mole fraction of ethanol and 11.92% mole fraction of water). Ethylene glycol and the azeotrope are stable and unstable nodes, respectively. Water and ethanol are two saddle points. As you drag the locator, the program plots the residue curve passing through the locator position. Such a residue curve map computation is useful in the design of extractive distillation columns for water-ethanol separations (i.e., ethanol dehydration) using ethylene glycol as an entrainer.
(88.08% mole fraction of ethanol and 11.92% mole fraction of water). Ethylene glycol and the azeotrope are stable and unstable nodes, respectively. Water and ethanol are two saddle points. As you drag the locator, the program plots the residue curve passing through the locator position. Such a residue curve map computation is useful in the design of extractive distillation columns for water-ethanol separations (i.e., ethanol dehydration) using ethylene glycol as an entrainer.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
For more information, see:
M. F. Doherty and M. F. Malone, Conceptual Design of Distillation Systems, New York: McGraw-Hill, 2001.