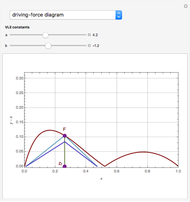
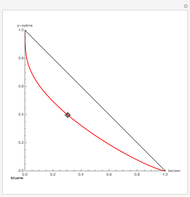
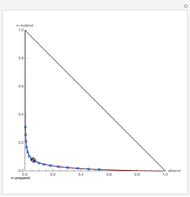
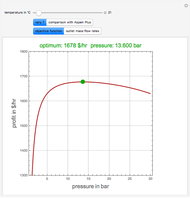
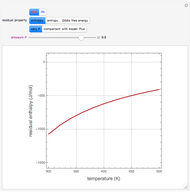
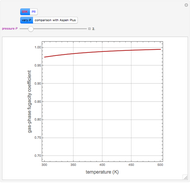
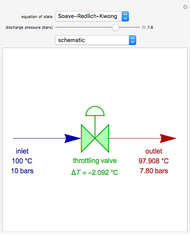
Residue Curve Map for a Non-Ideal Ternary Mixture with a Distillation Boundary

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
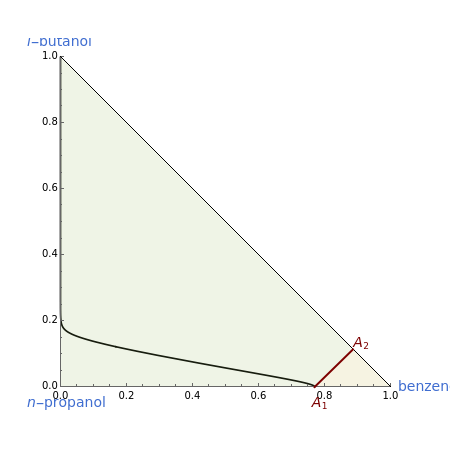
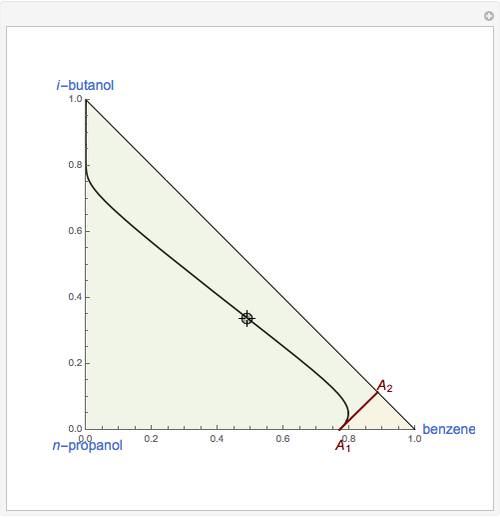
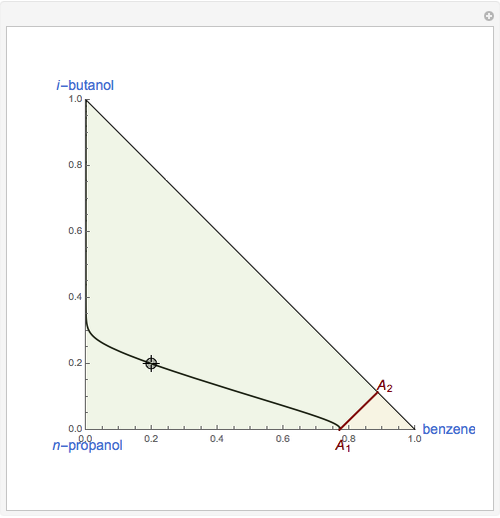
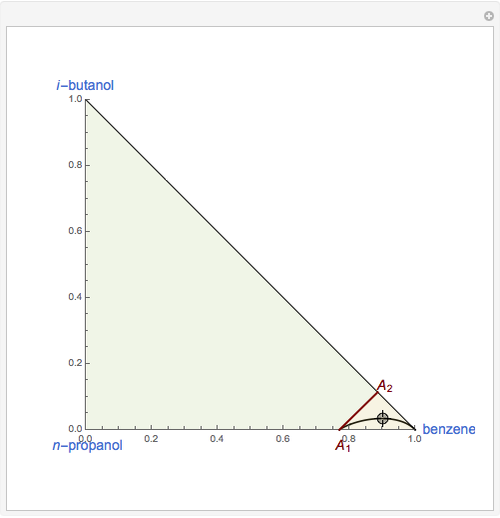
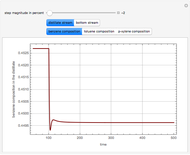
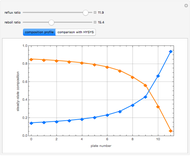
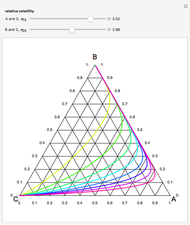
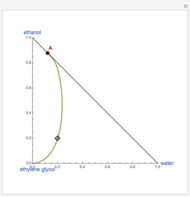
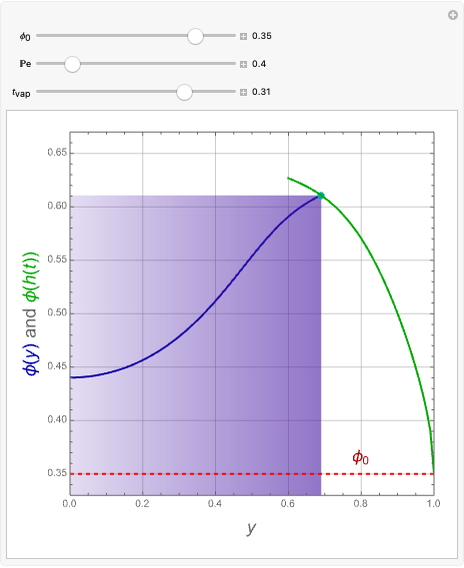
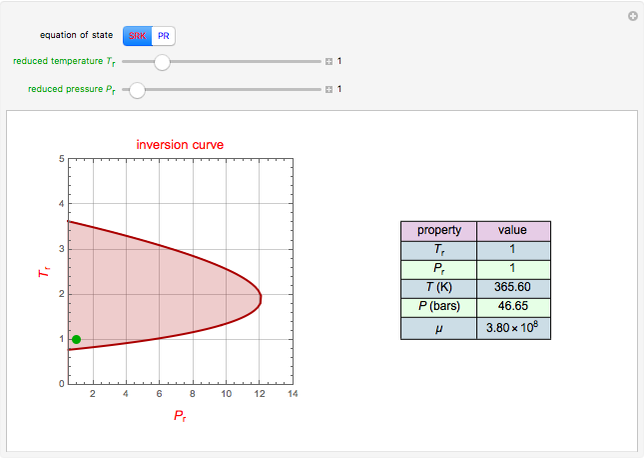
This Demonstration plots the residue curve of a non-ideal ternary mixture composed of benzene, 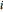 -butanol and
-butanol and  -propanol. Deviation from ideal behavior in the liquid phase is taken into account using the Wilson model in order to compute the activity coefficients. The total pressure is 101.325 kPa. This mixture presents two azeotropes: (1) between benzene and
-propanol. Deviation from ideal behavior in the liquid phase is taken into account using the Wilson model in order to compute the activity coefficients. The total pressure is 101.325 kPa. This mixture presents two azeotropes: (1) between benzene and  -propanol, marked in the ternary diagram by
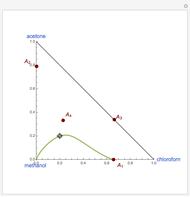
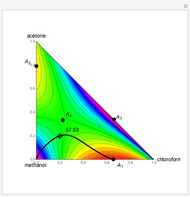
-propanol, marked in the ternary diagram by 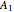 (77% mole fraction of benzene); (2) between benzene and
(77% mole fraction of benzene); (2) between benzene and 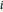 -butanol, marked in the ternary diagram by
-butanol, marked in the ternary diagram by 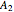 (88.62% mole fraction of benzene). This ternary mixture has two distillation regions (the yellow and green shaded areas) with a distillation boundary connecting the two binary azeotropes (the straight red segment in the diagram). Benzene and
(88.62% mole fraction of benzene). This ternary mixture has two distillation regions (the yellow and green shaded areas) with a distillation boundary connecting the two binary azeotropes (the straight red segment in the diagram). Benzene and 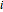 -butanol are stable nodes;
-butanol are stable nodes;  -propanol and
-propanol and 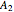 are two saddle points, and finally
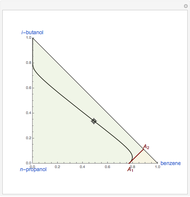
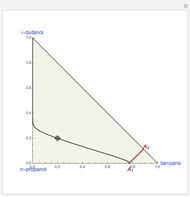
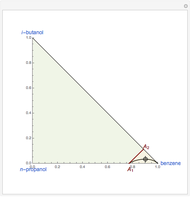
are two saddle points, and finally  is an unstable node. You can change the position of the locator to plot the residue curve passing at the locator position.
is an unstable node. You can change the position of the locator to plot the residue curve passing at the locator position.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
For more information, see
M. F. Doherty and M. F. Malone, Conceptual Design of Distillation Systems, New York: McGraw-Hill, 2001.