Reversible and Irreversible Isothermal Expansion of an Ideal Gas

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
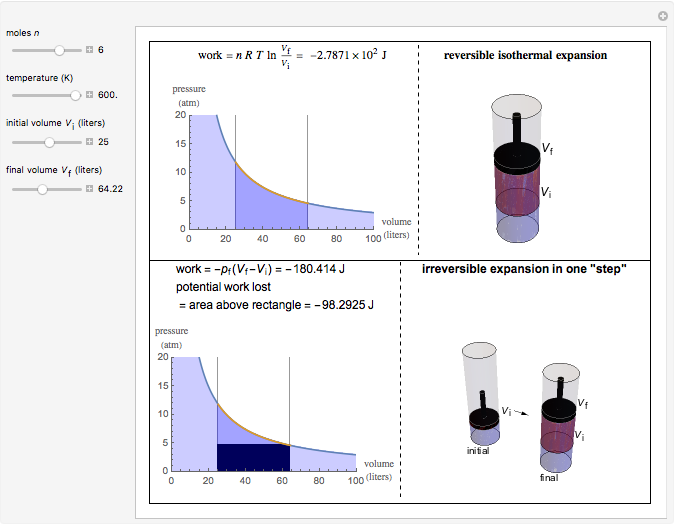
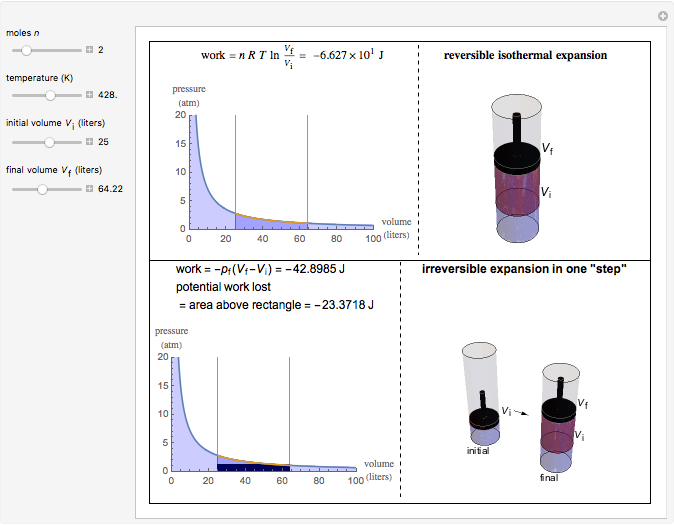
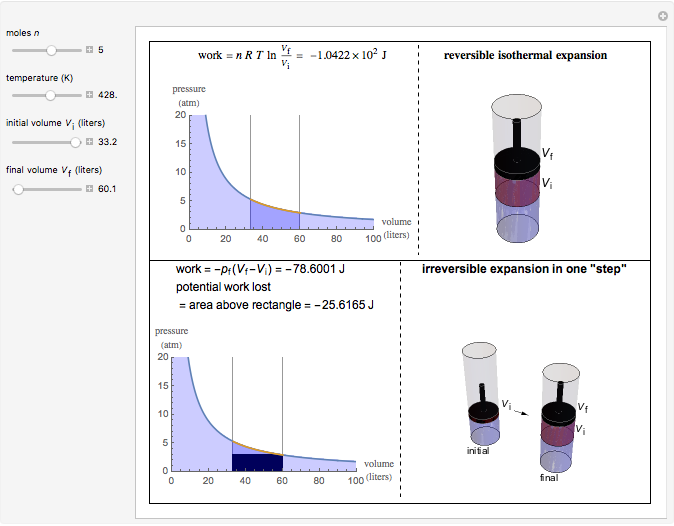
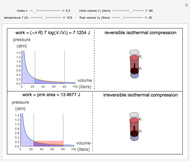
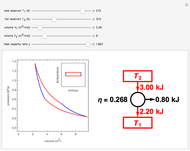
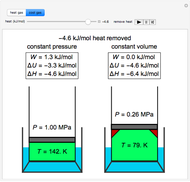
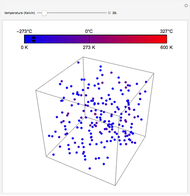
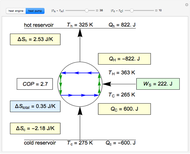
This Demonstration compares the thermodynamic processes of reversible and irreversible isothermal expansion of an ideal gas. The graph and the image of a piston at the top represent the slow expansion of a gas from an initial volume 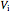 to a final volume
to a final volume 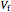 (you can vary these volumes with the sliders). Reversible work is given by the integral
(you can vary these volumes with the sliders). Reversible work is given by the integral 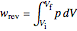 , which equals the lightly shaded area below the top curve. By the usual thermodynamic convention, negative work means work done by the system on the surroundings.
, which equals the lightly shaded area below the top curve. By the usual thermodynamic convention, negative work means work done by the system on the surroundings.
Contributed by: Blair Winograd (June 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] P. Atkins and L. Jones, Chemical Principles: The Quest for Insight, New York: W. H. Freeman, 1999.
Permanent Citation