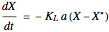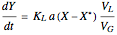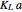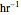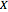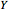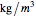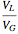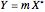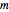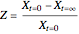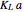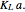Single Solute Batch Two-Phase Extraction

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
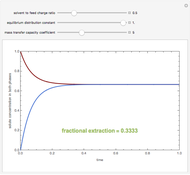
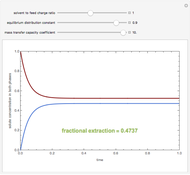
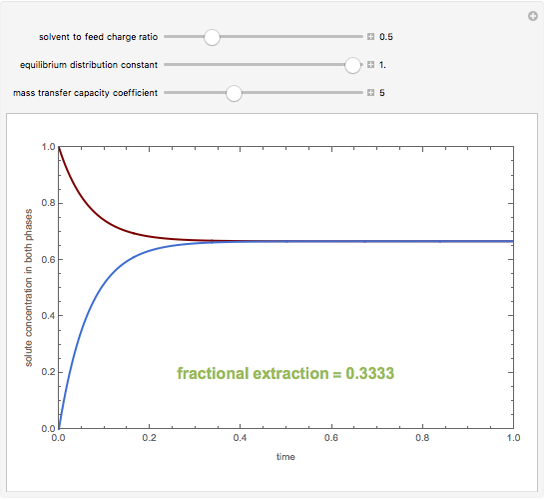
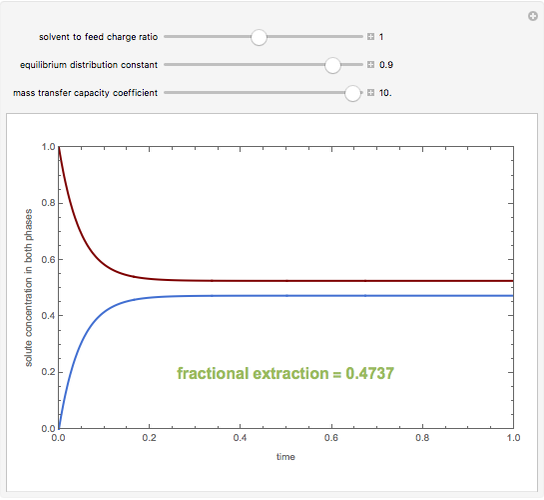
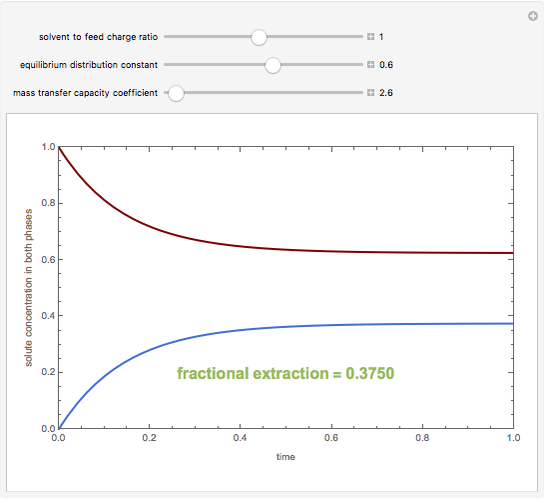
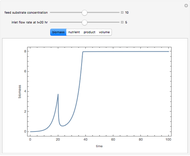
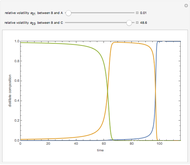
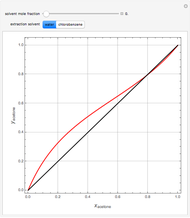
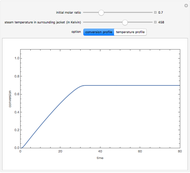
A single solute, 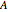 , is being transferred from a feed into an immiscible fresh solvent,
, is being transferred from a feed into an immiscible fresh solvent, 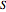 , in a batch two-phase extraction system. This Demonstration displays the dynamic approach to equilibrium for values of the linear equilibrium distribution constant, the solvent to feed-charge ratio, and the mass-transfer capacity coefficient, all to be set by the user.
, in a batch two-phase extraction system. This Demonstration displays the dynamic approach to equilibrium for values of the linear equilibrium distribution constant, the solvent to feed-charge ratio, and the mass-transfer capacity coefficient, all to be set by the user.
Contributed by: Housam Binous (September 2008)
Open content licensed under CC BY-NC-SA
Snapshots
Details
J. Ingham, I. J. Dunn, E. Heinzle, and J. E. Prenosil, Chemical Engineering Dynamics, 2nd ed., Weinheim, Germany: Wiley-VCH, 2000 pp. 494–496.
Permanent Citation