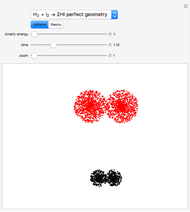
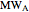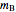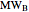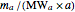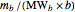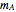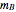This Demonstration shows how to calculate, for a simple theoretical chemical reaction with just two reactants: 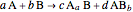 , the excess and the limitant reactants. The stoichiometric coefficients
, the excess and the limitant reactants. The stoichiometric coefficients  and
and  have been calculated by satisfying the mass balance.
have been calculated by satisfying the mass balance.
Chemical reaction equations give the ideal stoichiometric relationship among reactants and products. However, the given reactants are not necessarily a stoichiometric mixture. Reactants that are not used up in the completed chemical reaction are called excess reagents. The reagent that is completely used up is called the limiting reagent, because its quantity limits the quantity of product. The excess or the limitation depend on the following parameters: mass 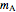 (g), molecular weight
(g), molecular weight 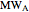 (g/mol) and stoichiometric coefficient
(g/mol) and stoichiometric coefficient  of the first reagent; and mass
of the first reagent; and mass 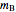 (g), molecular weight
(g), molecular weight 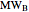 (g/mol) and stoichiometric coefficient
(g/mol) and stoichiometric coefficient  of the second reagent.
of the second reagent.
In order to determine the limiting reagent in a reaction like 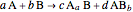 that is already balanced, you need to do the following:
that is already balanced, you need to do the following:
1. Convert all the given information into moles by dividing the mass by the product of the molecular weight and the corresponding stoichiometric coefficient: 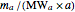 and
and 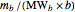 .
.
2. Compare the two results. The limiting reactant is the one with the lowest value. When these two values are the same, the reactants have the proper proportion and the reaction runs to completion with no limiting or excess reagents [1].
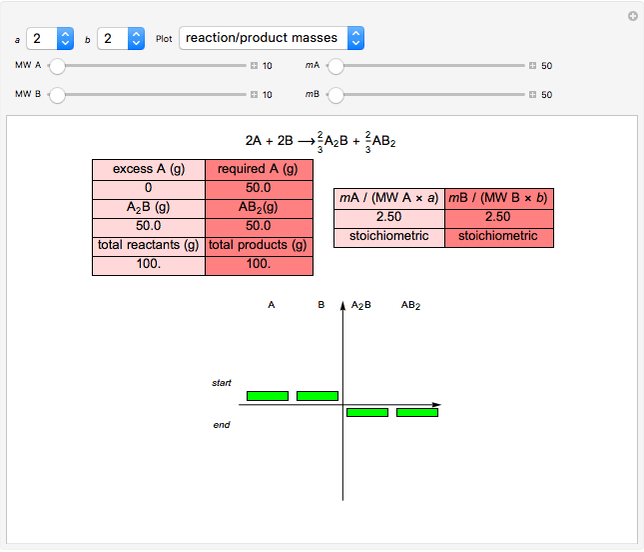
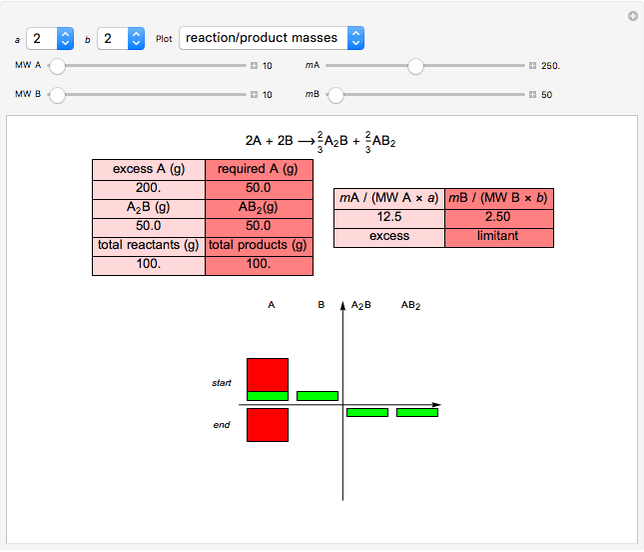
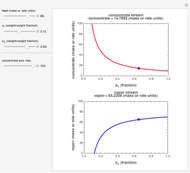
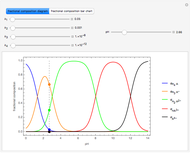
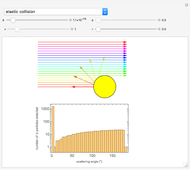
The left grid shows the excess quantity and the reactant that has actually been used up. The bottom row verifies the conservation of mass by showing that the sum of reactant masses (excluding any excess quantity) equals the sum of the product masses.
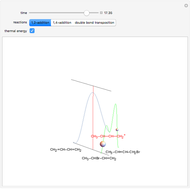
The first plot displays the quantities of reactants and products both at the start and at the end of the reaction, proportional to the areas of rectangles; green rectangles represent the amounts of reactants used and products formed, giving the chemical formulas. The quantities at the start are represented in the top half: the reactants on the right and the products on the left, the latter not being present at the start (nothing is displayed in the first quadrant), and any excess quantities being shown (in red in case of excess of A or in blue in case of excess of B). The bottom half shows the quantities at the end of the reaction: any excess reactant on the left (in red in case of excess of A or in blue in case of excess of B) and the products on the right. In case of stoichiometric mixture, obviously no excess is present and nothing is displayed in the third quadrant.
The plots, which can be selected through the control, show the relationship between the excess reactants and the parameters 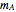 ,
, 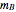 ,
,  ,
,  ,
, 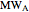 ,
, 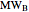 . In this Demonstration, the excess of A is represented in red with positive ordinate value, while the excess of B is represented in blue with negative ordinate value.
. In this Demonstration, the excess of A is represented in red with positive ordinate value, while the excess of B is represented in blue with negative ordinate value.
[less]

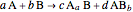 , the excess and the limitant reactants. The stoichiometric coefficients
, the excess and the limitant reactants. The stoichiometric coefficients  and
and  have been calculated by satisfying the mass balance.
have been calculated by satisfying the mass balance.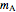 is shown.
is shown.