Study of a Four-Stage Batch Distillation Column

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
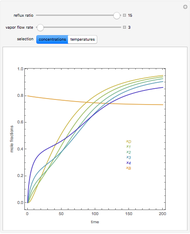
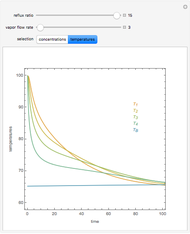
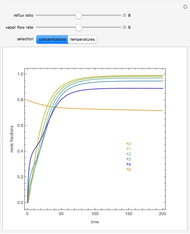
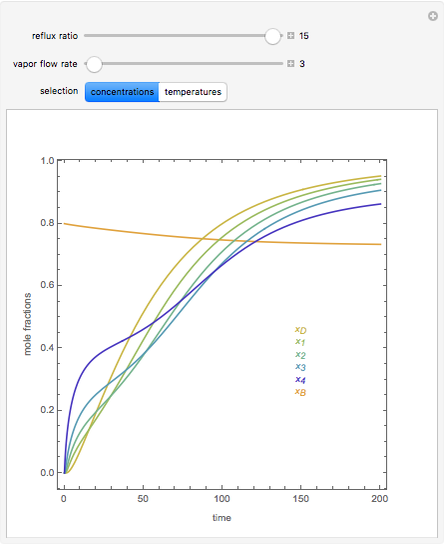
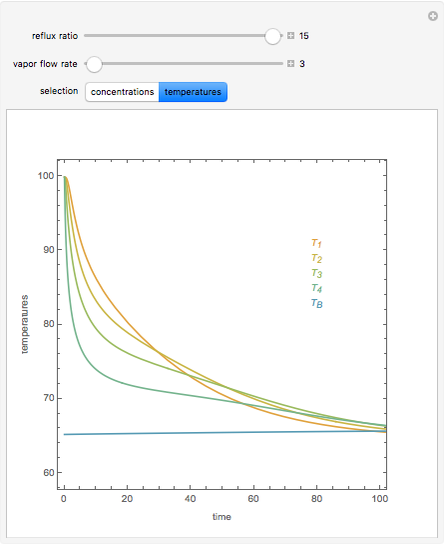
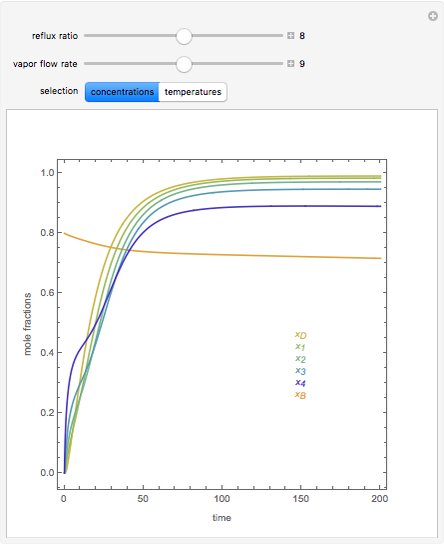
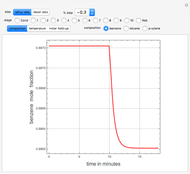
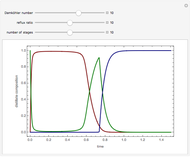
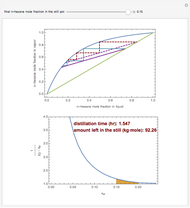
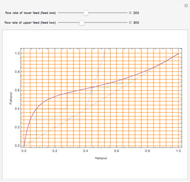
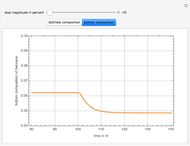
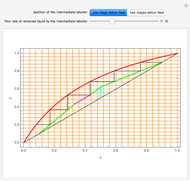
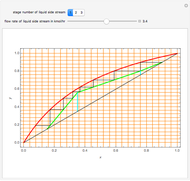
In this theoretical Demonstration, a batch column with four plates is used to separate a binary mixture of water and methanol at 1 bar. The Van Laar equation is chosen to estimate the activity coefficients because the binary mixture water-methanol deviates from the ideal behavior in the liquid phase. At each plate of the column, temperature and concentration profiles versus time are plotted for reflux ratios and vapor flow rates set by the user. The variables 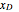 and
and 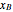 are the mole fractions in the distillate and the bottom of the still, respectively;
are the mole fractions in the distillate and the bottom of the still, respectively; 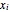 and
and 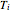 are the mole fraction and temperature at stage
are the mole fraction and temperature at stage 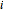 ,
,  1 to 4; and finally,
1 to 4; and finally, 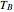 is the temperature in the still. The top stage is stage one and the bottom stage is stage four. This Demonstration uses NDSolve to treat a complex system of 13 algebraic-differential equations.
is the temperature in the still. The top stage is stage one and the bottom stage is stage four. This Demonstration uses NDSolve to treat a complex system of 13 algebraic-differential equations.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
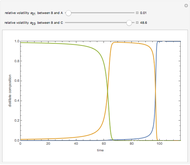
The Van Laar equation estimates the activity coefficient:
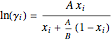 ,
,
where 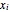 is the mole fraction of component
is the mole fraction of component 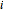 ,
, 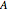 and
and 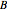 are binary parameters, and
are binary parameters, and 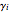 is the activity coefficient.
is the activity coefficient.
J. Ingham, I. J. Dunn, E. Heinzle, and J. E. Prenosil, "BUBBLE - Bubble Point Calculation for a Bath Distillation Column," Chemical Engineering Dynamics, 2nd ed., Weinheim, Germany: Wiley-VCH, 2000 pp. 559–564.
Permanent Citation