Titration of Weak Acids with Strong Bases

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
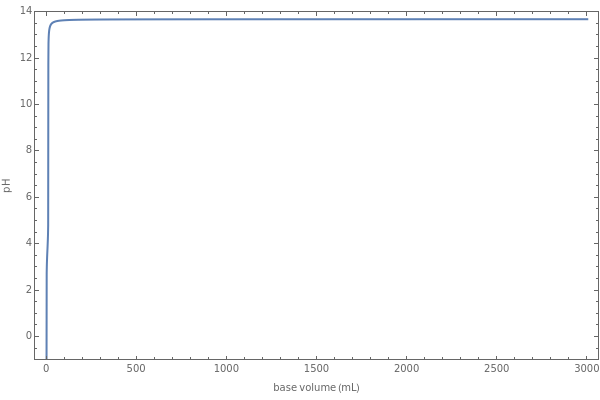
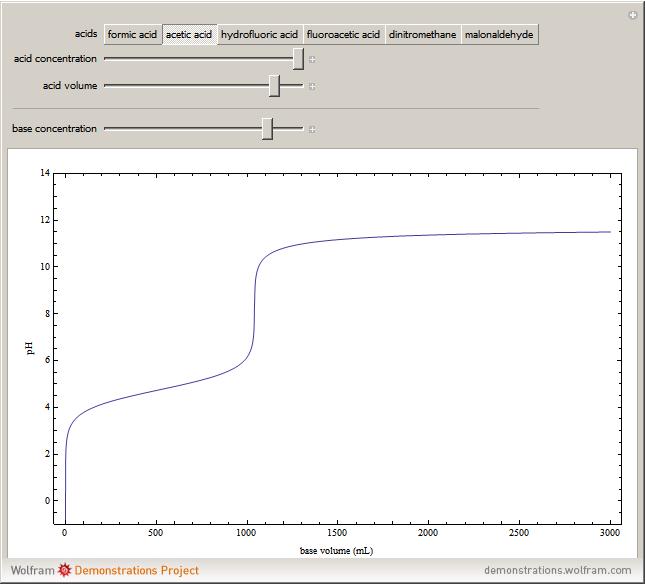
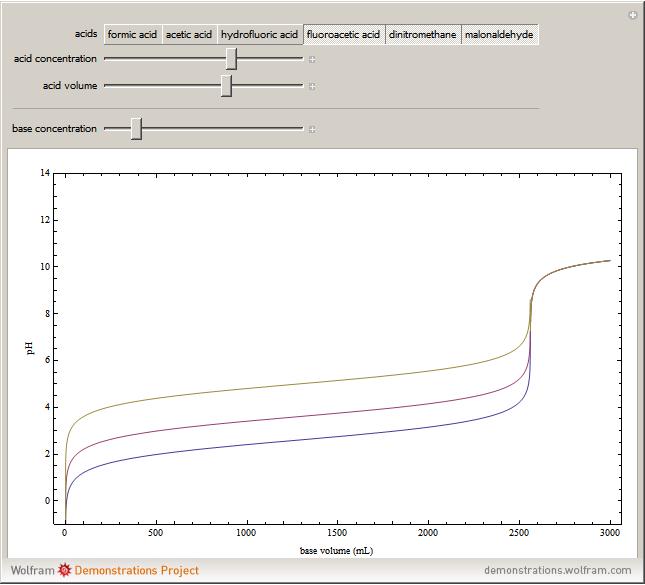
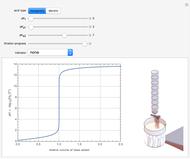
Weak monoprotic acids can be neutralized in the presence of a strong base (such as sodium hydroxide) to form a buffered solution between the excess acid and the newly formed sodium salt of the conjugate base. As base is added, but before the acid is completely neutralized, the  of the solution can be calculated using the Henderson–Hasselbalch equation:
of the solution can be calculated using the Henderson–Hasselbalch equation: , where
, where  is the negative log of the acid dissociation constant and
is the negative log of the acid dissociation constant and  and
and  are concentrations in moles per liter. After the neutralization, the
are concentrations in moles per liter. After the neutralization, the  is determined by the amount of excess base present. The volume of base required to reach the equivalence point (point of inflection) is determined by three variables: the concentration and volume of acid present and the concentration of base being added.
is determined by the amount of excess base present. The volume of base required to reach the equivalence point (point of inflection) is determined by three variables: the concentration and volume of acid present and the concentration of base being added.
Contributed by: Kristen Aramthanapon and Wiktor Macura (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
K. W. Whitten, R. E. Davis, and M. L. Peck, General Chemistry, 6th ed., Fort Worth, TX: Saunders College Publishing, 2000.
Permanent Citation
"Titration of Weak Acids with Strong Bases"
http://demonstrations.wolfram.com/TitrationOfWeakAcidsWithStrongBases/
Wolfram Demonstrations Project
Published: March 7 2011