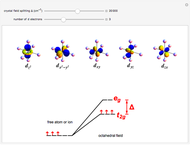
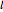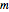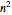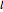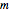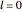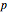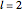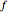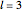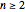1. Mendeleev Interpreted by Schrödinger and Lewis

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
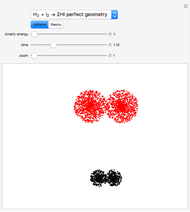
This Demonstration shows an implementation of P. A. M. Dirac's prophecy: "Chemistry will become an exercise of applied math".
[more]
Contributed by: D. Meliga, A. Ratti, L. Lavagnino and S. Z. Lavagnino (November 2019)
Open content licensed under CC BY-NC-SA
Details
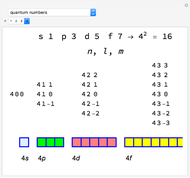
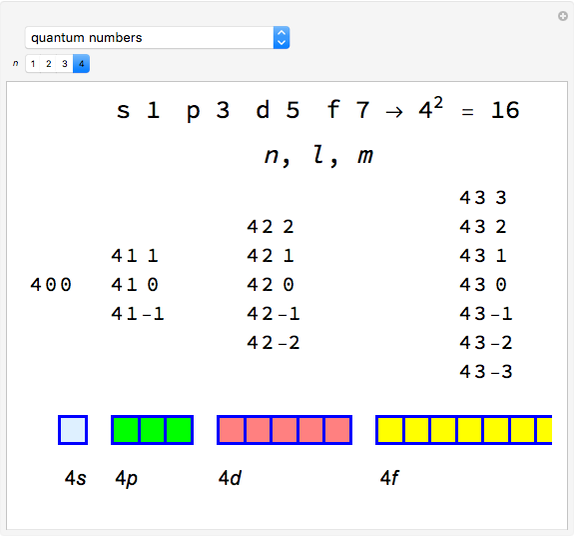
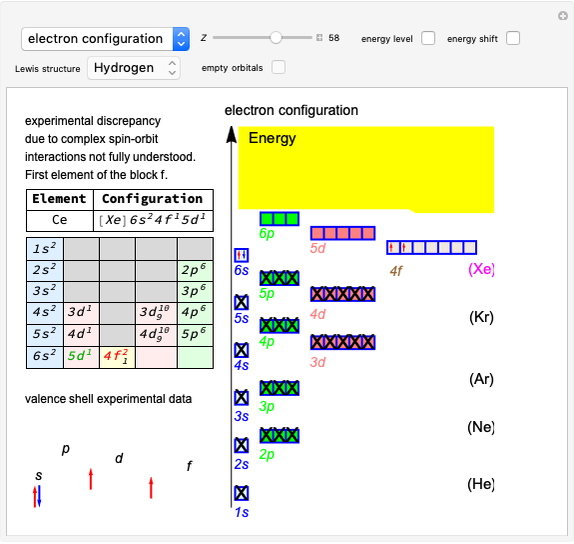
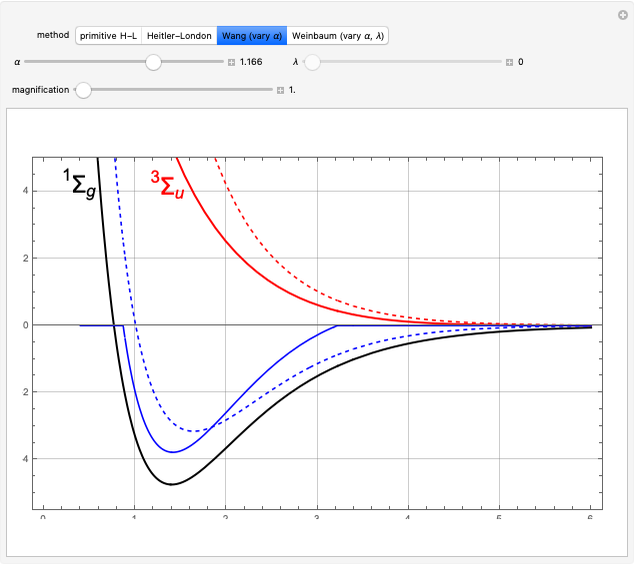
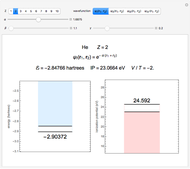
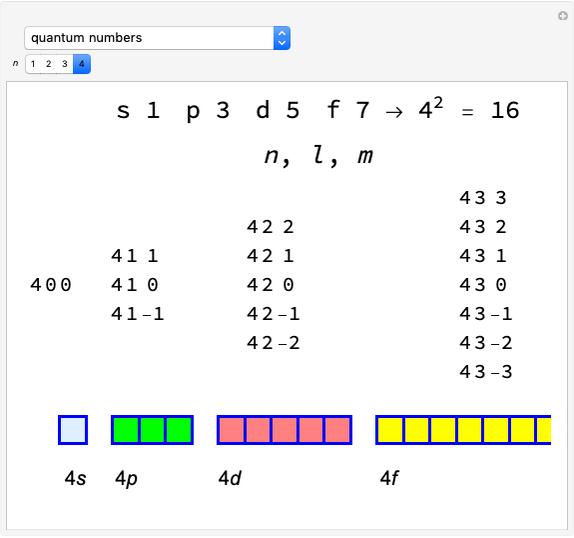
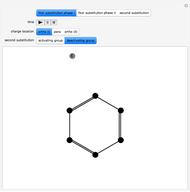
Snapshot 1: orbital quantum numbers: principal ( ), orbital angular momentum (
), orbital angular momentum (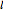 ) and spin magnetic (
) and spin magnetic (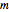 ); the sum of the odd numbers of each subshell is equal to
); the sum of the odd numbers of each subshell is equal to 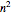
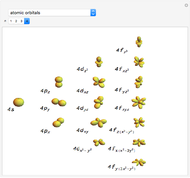
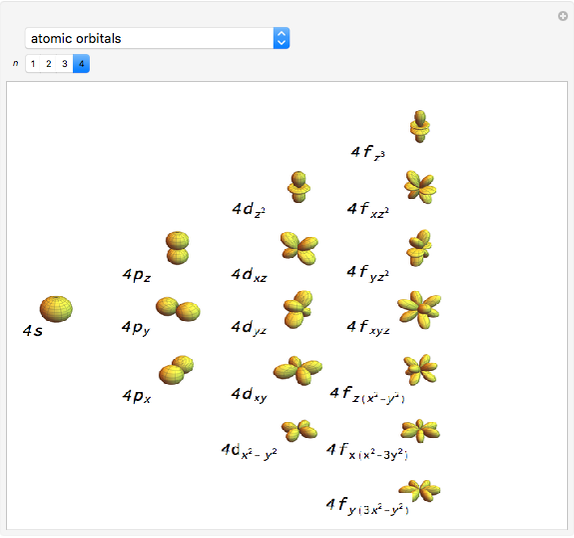
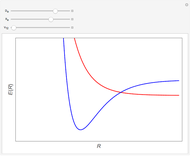
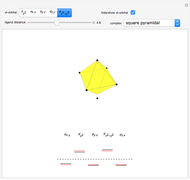
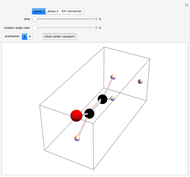
Snapshot 2: orbital shapes with 
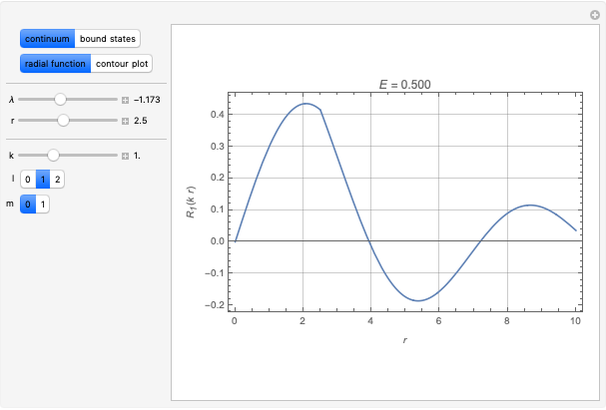
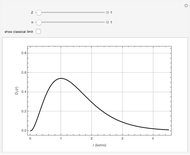
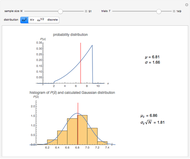
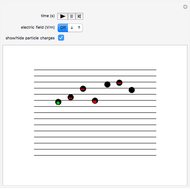
Snapshot 3: radial probability of each orbital with 
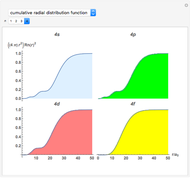
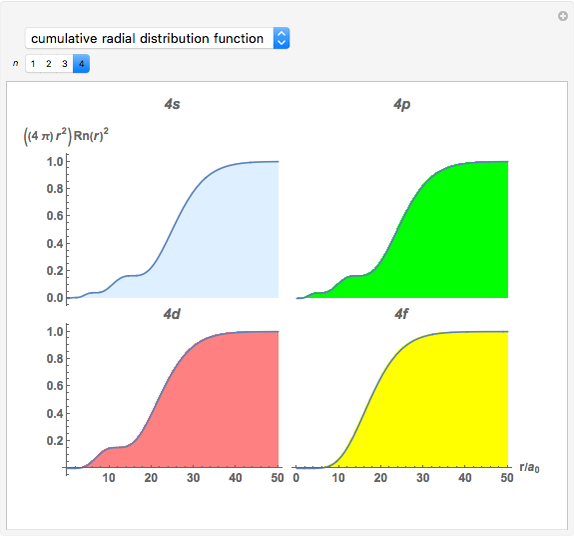
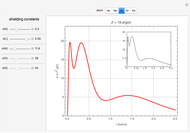
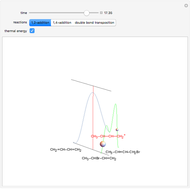
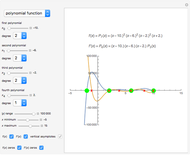
Snapshot 4: cumulative radial probability of each orbital with  ; each cumulative probability tends to 1 as every single probability is normalized
; each cumulative probability tends to 1 as every single probability is normalized
Reference
[1] L. Lua, A. Iskandar and M. Magsombol. "Electron Configuration." (Sep 17, 2019) chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/10%3 A_Multi-electron_Atoms/Electron_Configuration.
Snapshots
Permanent Citation