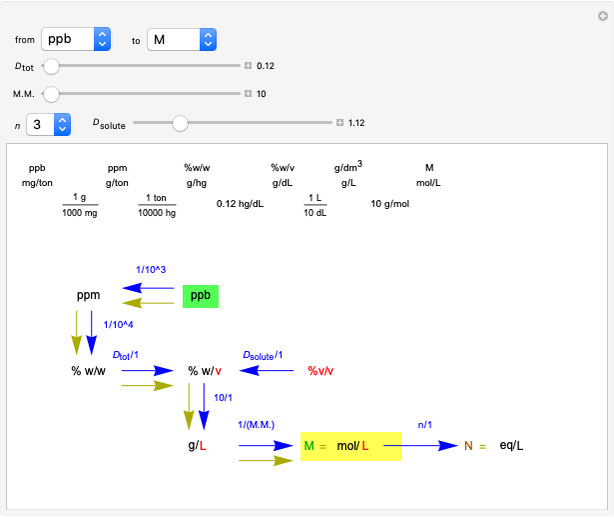
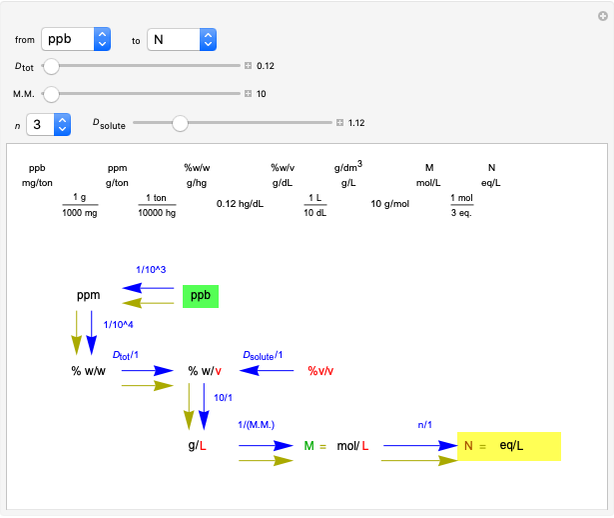
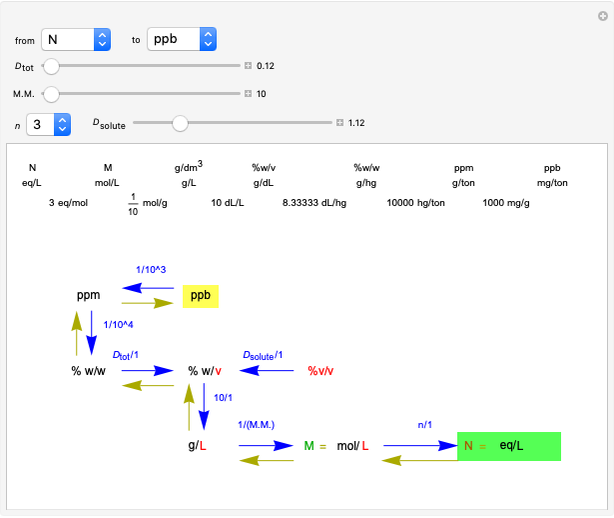
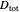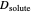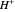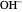Common Units for Measuring Concentrations

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
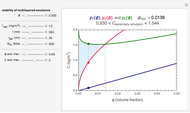
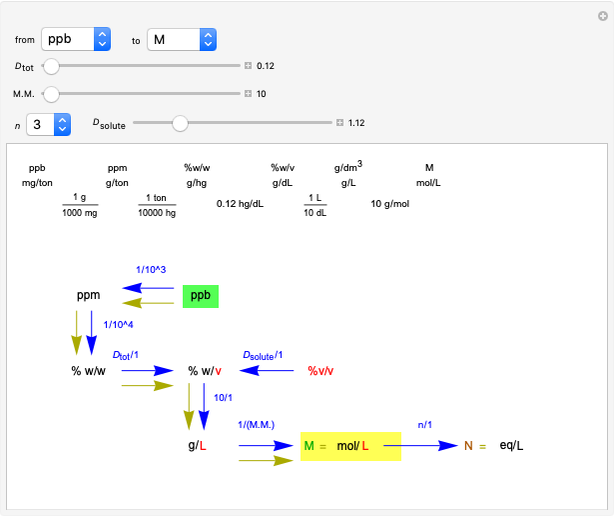
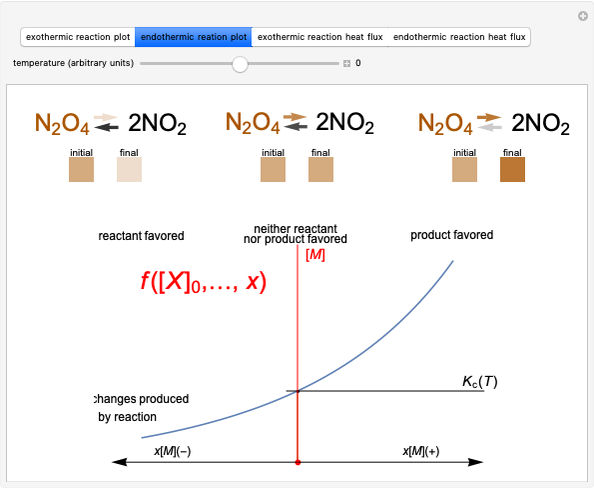
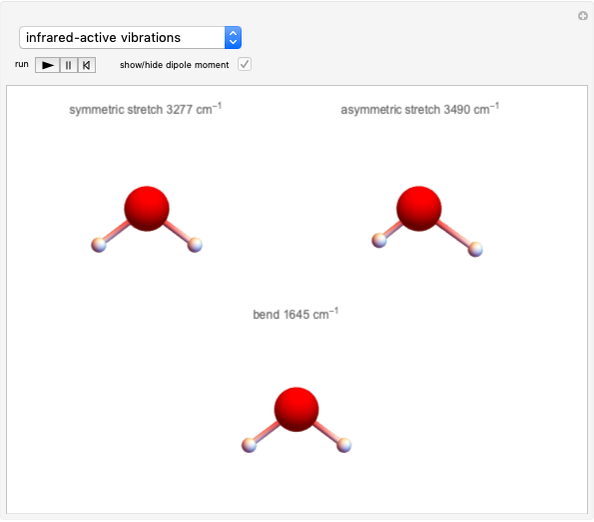
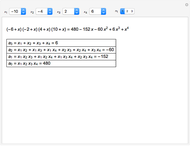
This Demonstration shows how to change units of a concentration by using appropriate conversion factors. The most common units are considered, except for mole fraction and molality, where there are no conversion factors. Initial and final units are given by clicking "from" and "to."
[more]
Contributed by: D. Meliga, L. Lavagnino and S. Z. Lavagnino (December 2020)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: conversion factors from parts per billion (ppb) to normality (N)
Snapshot 2: conversion factors from normality (N) to parts per billion (ppb)
Snapshot 3: conversion factors from parts per billion (ppb) to volume by volume (%v/v)
Reference
[1] P. M. Lausarot and G. A.Vaglio, Stechiometria per la Chimica generale, Padova, Italy: Piccin-Nuova Libraria S.p.A., 2005.
Permanent Citation