Gas Absorption Computed Using the Gauss-Seidel Method

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
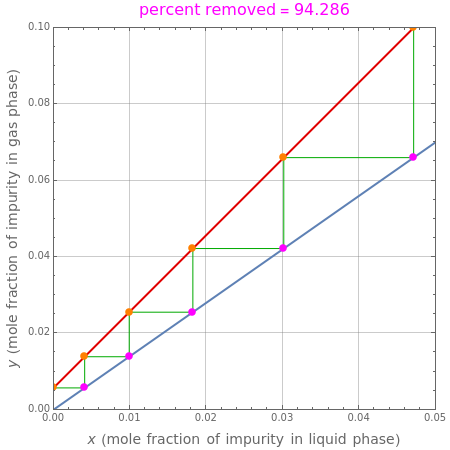
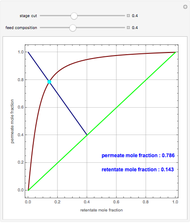
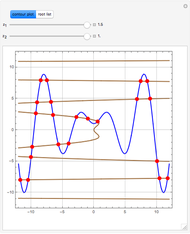
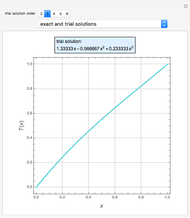
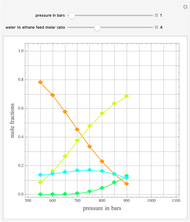
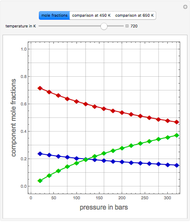
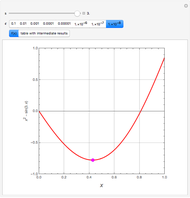
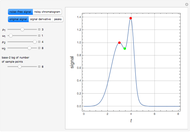
Consider a tray absorption column used to remove an impurity from a gas stream. A pure solvent is used for this absorption operation. The solvent molar flow rate  and the gas molar flow rate
and the gas molar flow rate  are considered constant (i.e., the dilute system hypothesis holds). You can set the number of equilibrium stages, the value of the slope
are considered constant (i.e., the dilute system hypothesis holds). You can set the number of equilibrium stages, the value of the slope  of the equilibrium line (
of the equilibrium line ( ), the solvent-to-gas molar flow rate ratio (
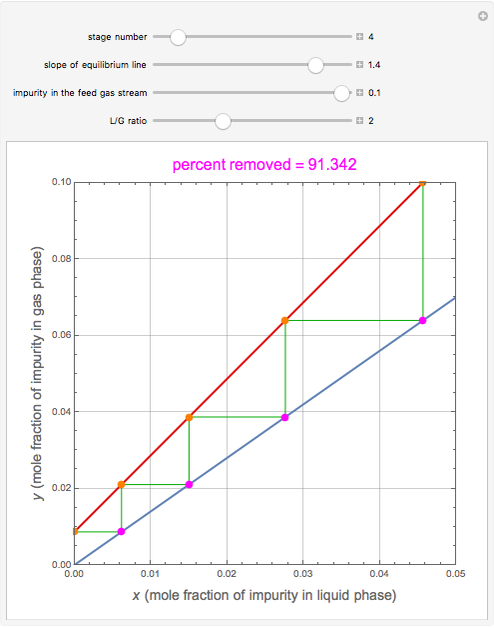
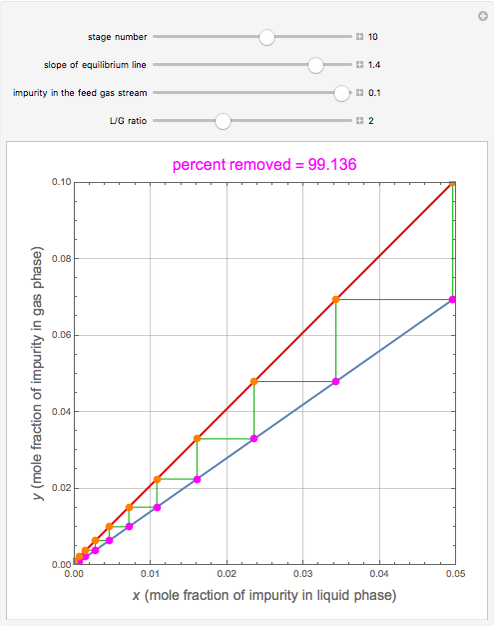
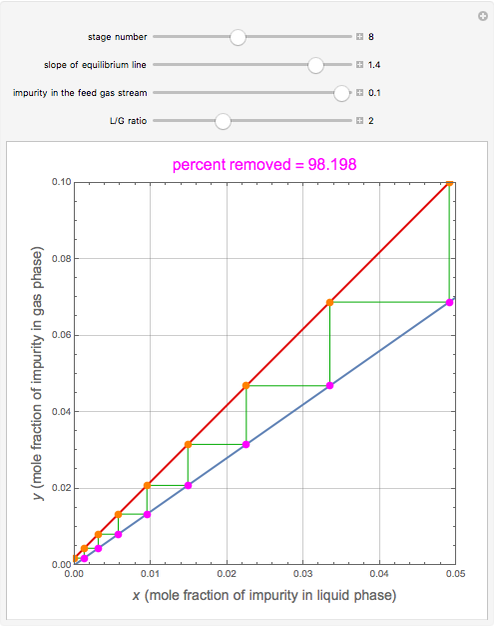
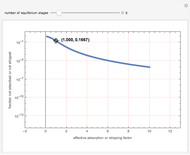
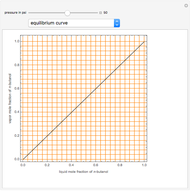
), the solvent-to-gas molar flow rate ratio ( , and the mole fraction of the impurity in the gas fed to the absorption column. This Demonstration computes the McCabe–Thiele diagram. The horizontal green lines represent the theoretical equilibrum stages in the absorption column. The percent removal of the impurity for the specified conditions is displayed on the plot.
, and the mole fraction of the impurity in the gas fed to the absorption column. This Demonstration computes the McCabe–Thiele diagram. The horizontal green lines represent the theoretical equilibrum stages in the absorption column. The percent removal of the impurity for the specified conditions is displayed on the plot.
Contributed by: Housam Binous and Brian G. Higgins (March 2012)
Open content licensed under CC BY-NC-SA
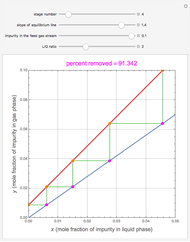
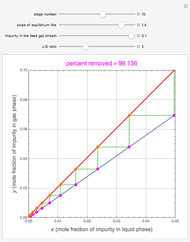
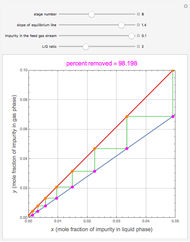
Snapshots
Details
The approach used in this Demonstration is based on the solution of a system of linear equations, which can be written in matrix form for  , where
, where  is the number of stages of the absorption column, as follows:
is the number of stages of the absorption column, as follows:

 =
=  .
.
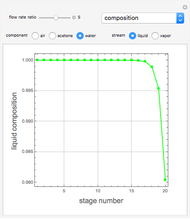
Taking advantage of the banded form of the matrix, one can use the Gauss–Seidel iterative technique to get the liquid compositions in all stages  with
with  .
.
Reference
[1] P. C. Wankat, Separation Process Engineering: Includes Mass Transfer Analysis, 3rd ed., Upper Saddle River, NJ: Pearson, 2012.
Permanent Citation