Oil-Drop Experiment

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
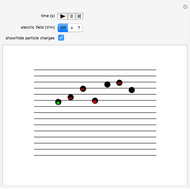
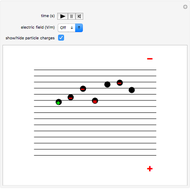
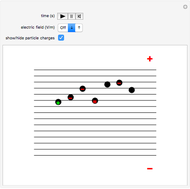
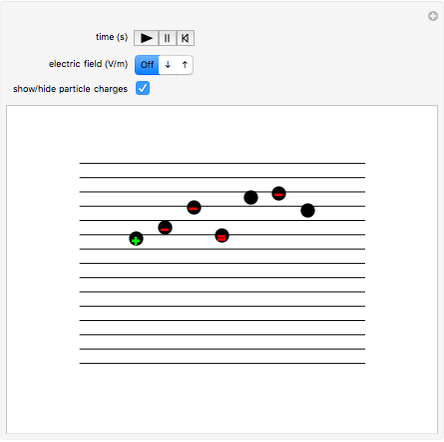
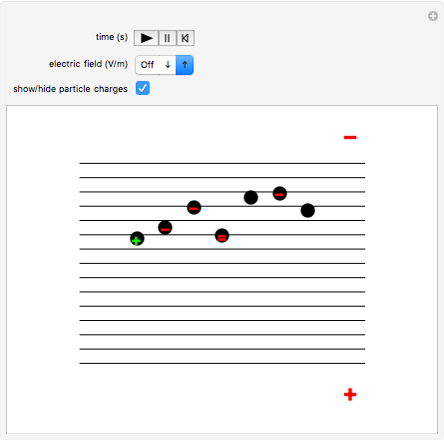
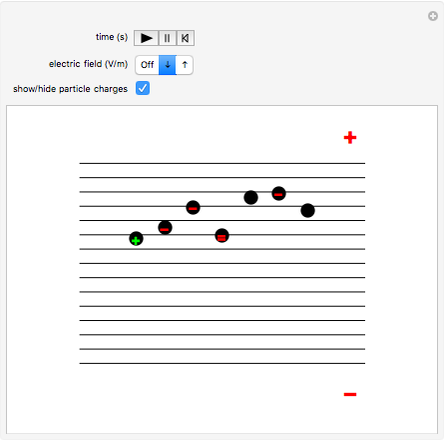
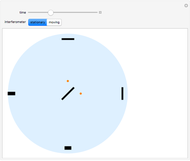
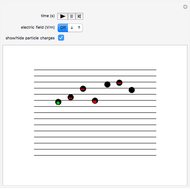
This Demonstration simulates Millikan's well-known oil-drop experiment. By means of a thin nozzle, atomized oil drops are sprayed between two parallel horizontal metal surfaces that form the plates of a capacitor. The drops are ionized using an external x-ray source; statistically, the most likely event is the capture of an electron by the oil drop. However, a drop can also wind up with no charge, a double negative charge or a positive charge. You can see the charges by checking the "show/hide particle charges" box.
[more]
Contributed by: D. Meliga and S.Z. Lavagnino (December 2017)
Additional contribution by: A. Ratti
Open content licensed under CC BY-NC-SA
Details
The equation of motion of an oil drop immersed in a fluid in the presence of a gravitational field and an electric field is given by
 ,
,
where  is the mass calculated through the oil density of the drop,
is the mass calculated through the oil density of the drop,  is the electronic charge,
is the electronic charge,  is gravitational acceleration,
is gravitational acceleration,  is the drag force due to air friction (in the case of low velocities, it depends linearly on velocity
is the drag force due to air friction (in the case of low velocities, it depends linearly on velocity  ),
),  is the electric field force (negative when the field is upward, positive when the field is downward), and
is the electric field force (negative when the field is upward, positive when the field is downward), and  is the buoyancy force. Solving the differential equation yields that the drop reaches a drift velocity
is the buoyancy force. Solving the differential equation yields that the drop reaches a drift velocity  , where
, where  and
and  is the initial velocity.
is the initial velocity.
To a good approximation, the drift velocity is reached after a time  ; this Demonstration assumes that drift velocity has already been reached.
; this Demonstration assumes that drift velocity has already been reached.
The measurement of  allowed Millikan to calculate the elementary electron charge, about
allowed Millikan to calculate the elementary electron charge, about  coulombs [3].
coulombs [3].
Snapshot 1: only the gravitational field is present; all drops move downward at the same velocity
Snapshot 2: added to the gravitational field, an upward electric field dominates for particles with a positive charge
Snapshot 3: the upward electric field also dominates for particles with a negative charge
References
[1] A. Redfield and F. Friedman. The Millikan Experiment [Video]. (Dec 13, 2017) www.youtube.com/watch?v=JuXtk3bC2iM.
[2] Wikipedia. "Oil Drop Experiment." (Dec 13, 2017) en.wikipedia.org/wiki/Oil_drop_experiment.
[3] Encyclopaedia Britannica. "Millikan Oil-Drop Experiment." (Dec 13, 2017) www.britannica.com/science/Millikan-oil-drop-experiment.
Snapshots
Permanent Citation