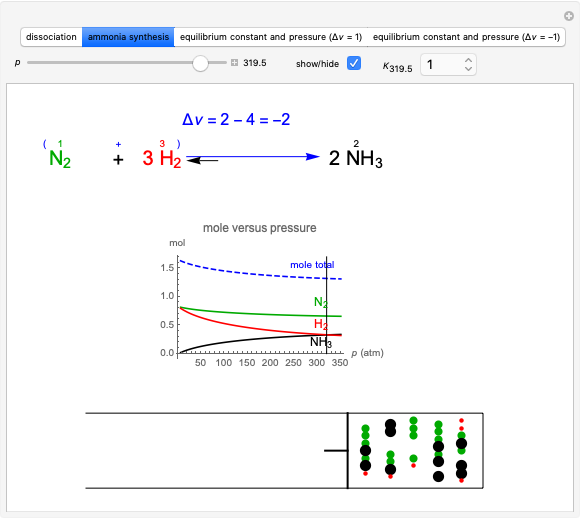
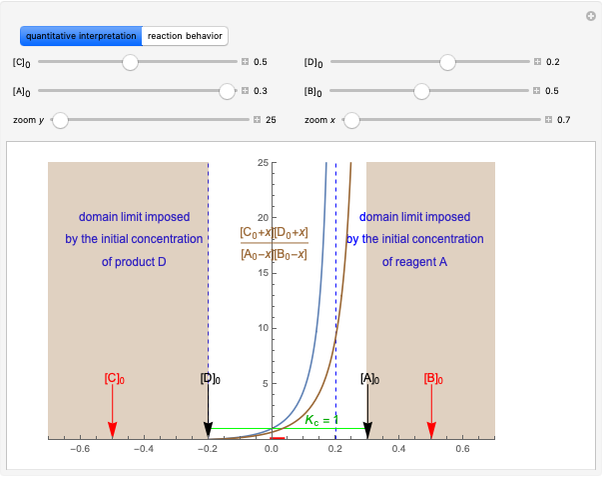
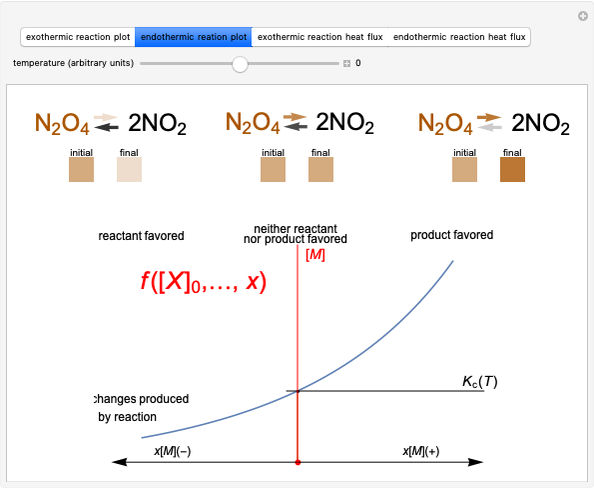
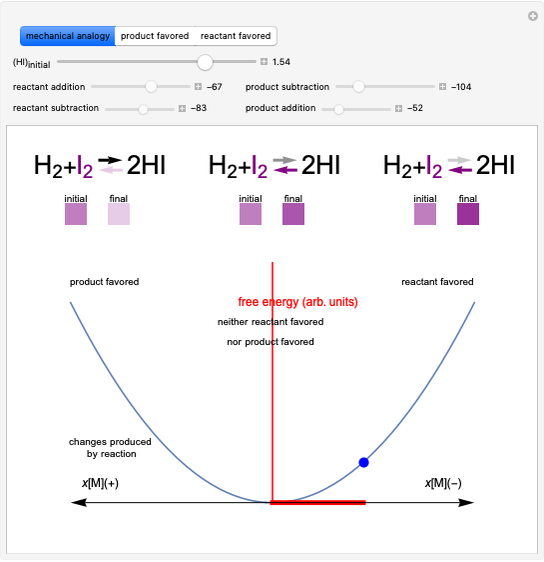
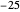Producing Extra-Dry Asti Spumante

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
"Aste nitet mundo Sancto custode Secundo" ("Asti shines in the world thanks to its patron saint Secundus")—this motto is on the coat of arms of the municipality of Asti); in particular, with Google Earth you can admire the verdant splendor of the Asti vineyards, in which the iconic Asti Spumante is produced. This Demonstration examines the complex process that transforms a wine into a sparkling wine [1].
[more]
Contributed by: D. Meliga, A. Ratti, L. Lavagnino and S. Z. Lavagnino (January 2021)
Additional contribution by: F. Bergamasco (Enologist)
Open content licensed under CC BY-NC-SA
Snapshots
Details
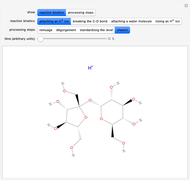
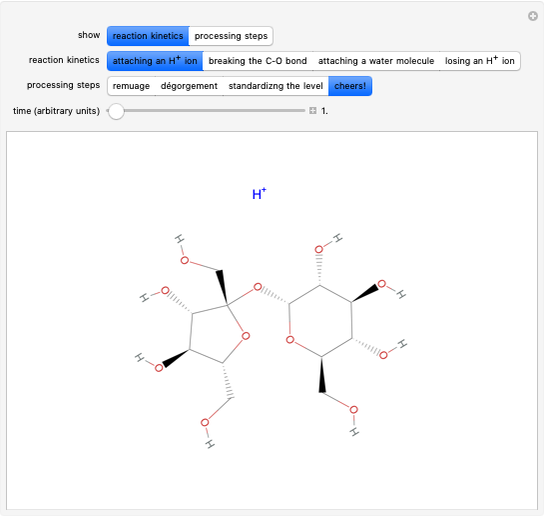
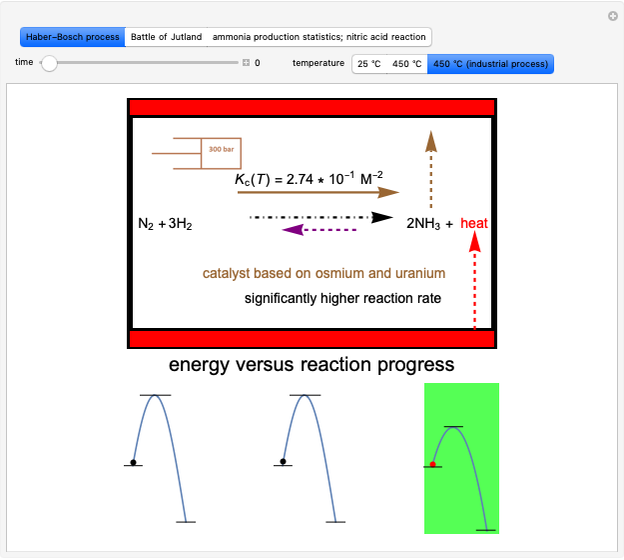
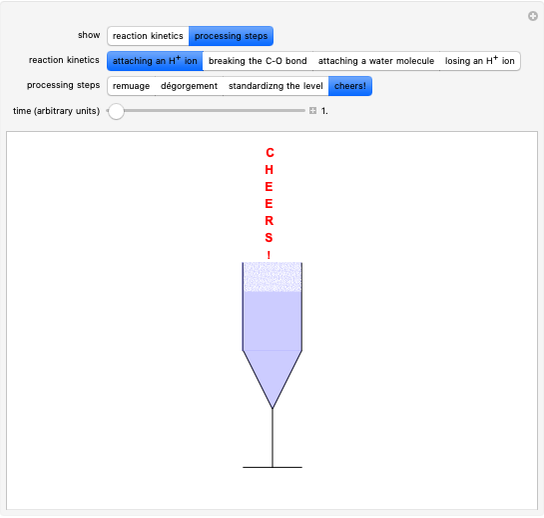
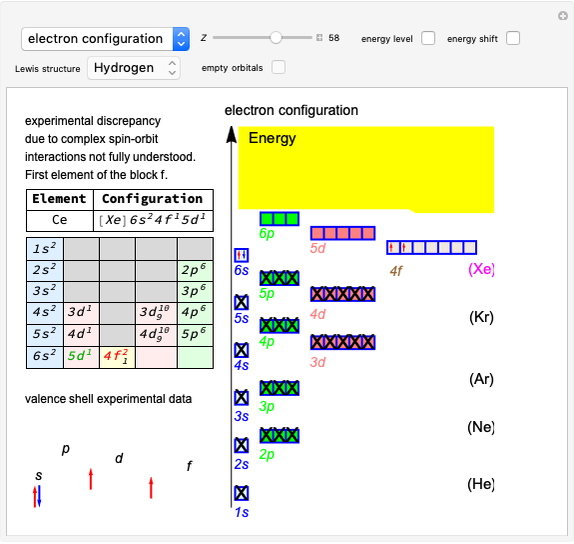
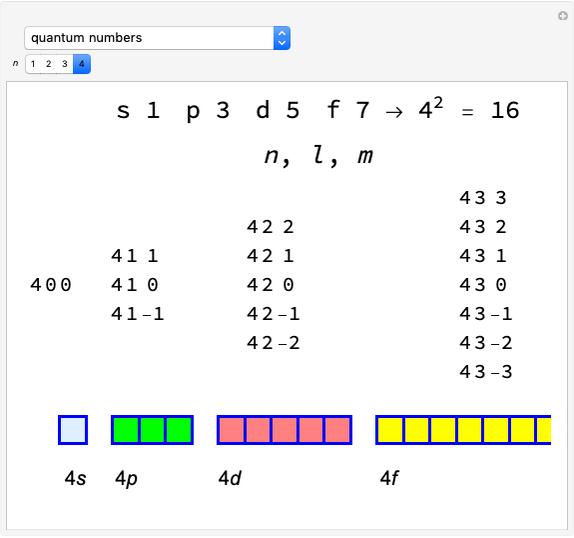
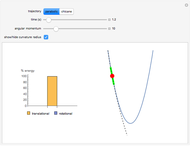
Snapshot 1: fast stage of the reaction mechanism: 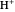 ion attachment with catalyst
ion attachment with catalyst 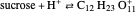
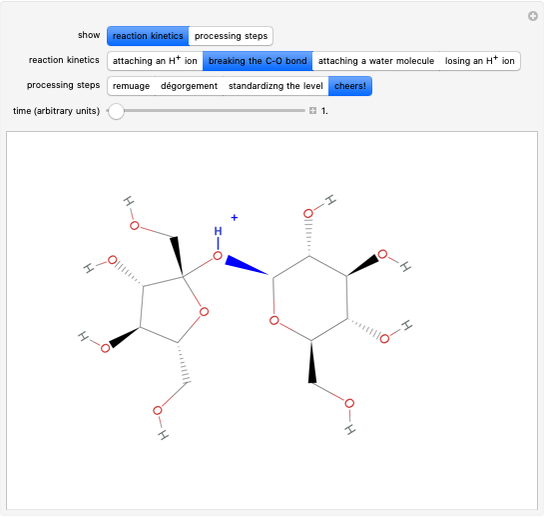
Snapshot 2: bottleneck (not because it happens in the bottle!): 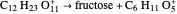
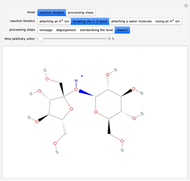
Snapshot 3: fast stage: water attachment: 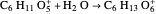
Snapshot 4: fast stage: 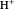 ion detachment (catalyst):
ion detachment (catalyst): 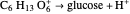
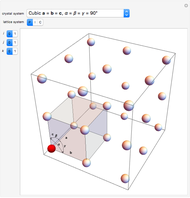
Snapshot 5: "remauge": the sediments (yeasts) produced by the second fermentation are made to slide in the neck of the bottle by means of a gradual increase from the horizontal position to the "sur pointe" position, all associated with small rotations of the bottle; the result is a perfectly clear liquid
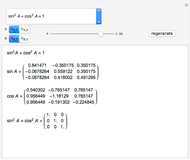
Snapshot 6: "dégorgement à la glace": with a quick opening of the cap, the internal pressure will expel the ice cylinder; the operation can be carried out either manually or mechanically
Snapshot 7: after adding and mixing the "liqueur d'éxpedition" and after the insertion of the "muselet," the bottles are returned to the cellar for a rest period
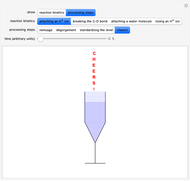
Snapshots 8: a flûte is a suitable glass for tasting dry sparkling wines: the rising bubbles and aromas in confined spaces prolong the effervescence of the sparkling wine while maintaining the classic "perlage" for longer; the traditional cup, coppa Asti, is used only for Asti Spumante dolce
References
[1] Wikipedia. "Asti Wine." (Dec 22, 2020) en.wikipedia.org/wiki/Asti_wine.
[2] R. T. Morrison and R. N. Boyd, Organic Chemistry, 3rd ed., Boston: Allyn and Bacon, 1973.
[3] "Reazione di idrolisi del saccarosio.doc." (Dec 15, 2020) puccini.chimica.uniba.it/didattica/corsi/labchimfis2/polarimetria.pdf.
Permanent Citation